Fragrances with note of lily of the valley
A fragrance and aroma technology, applied in the field of new compounds, can solve the problem of reproducing the impression of natural lily of the valley flower aroma and smell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0334] Embodiment 1: the synthesis of 4-isopropyl cyclohexanol (IX)
[0335]
[0336] 1 kg of 4-isopropylphenol (the above formula (X)), 20 g of ruthenium carbon and 2.5 L of ethanol were charged into the autoclave. 4-Isopropylphenol was hydrogenated at 140°C for 6 hours at a hydrogen pressure of 40 bar. After filtration and removal of solvent, 1.04 kg of crude product was obtained with a purity of 99.8% (cis / trans isomer ratio 1:1, quantitative yield).
Embodiment 2
[0337] Example 2: Synthesis of 8-isopropyl-4-oxaspiro[4.5]decan-3-one (VIII)
[0338]
[0339] A mixture of 768 g of 4-isopropylcyclohexanol (5.40 mol, 4.50 eq, formula (IX) above) and 1.90 g of di-tert-butyl peroxide (13.0 mmol, 0.01 eq) was heated to 140°C. At this temperature, a mixture of 257 g of 4-isopropylcyclohexanol (1.80 mol, 1.50 eq) and 103 g of methyl acrylate (1.20 mol, 1.00 eq) was added dropwise over 6 hours. The low-boiling products formed were removed. Then 6.5 g of di-tert-butyl peroxide (44.0 mmol, 0.04 equiv) was added and stirred at 160° C. for another 2 hours. After cooling, 150 g of 10% sodium sulfite solution was added to the reaction mixture, and stirred at 60° C. for 1 hour. The phases were separated and the organic phase was washed with 100 g of water. After removal of the solvent, the crude product was purified by distillation. 102 g of (3R)-3-isopropyl-6-methyl-7-oxabicyclo[4.1.0]heptane were isolated with a purity of 90% (cis / trans isome...
Embodiment 3
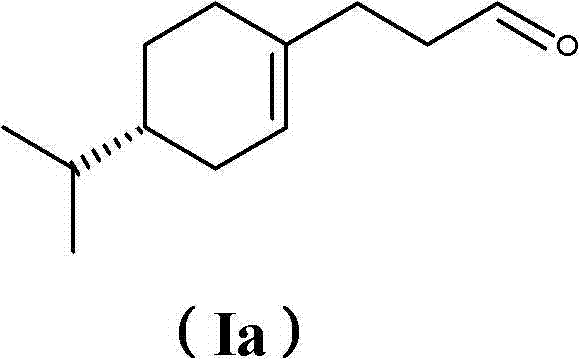
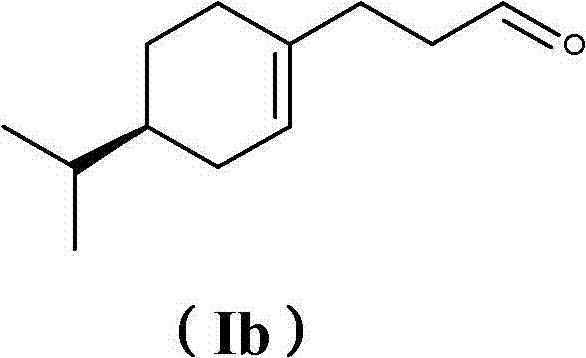
[0342] Example 3: Synthesis of 3-(4- Isopropylcyclohexen-1-yl)propanal (I)
[0343]
[0344] Catalyst preparation:
[0345] To a mixture of 34 g of cerium (III) acetate hydrate and 87 g of pumice was added 450 g of water. The mixture was stirred at 50 °C for 1 hour, then the water was removed in a rotary evaporator. A pyrolysis tube was filled with 35 g of prepared pumice (11 cm high) and heated at 440° C. for 1 hour.
[0346] Pyrolysis:
[0347] A mixture of 2 g of 8-isopropyl-4-oxaspiro[4.5]dec-3-one (93%, 9.5 mmol, formula (VIII) above) and 18 g of formic acid was charged at 440 °C in the presence of the above catalyst In the instrument, react in a nitrogen flow of 16 L / hour over 2 hours. The pyrolysis products were captured in a cooled trap, then diluted with MTBE and neutralized with saturated sodium bicarbonate solution. The phases were separated and the solvent was removed to obtain 1.5 g of crude product with a purity of 32%. This corresponds to a crude yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com