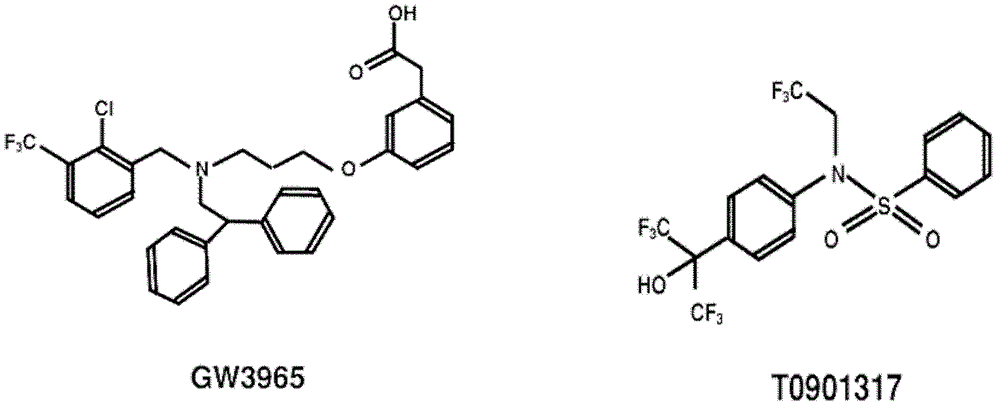
Application of cellular target liver X receptor in preparation of drugs treating hepatitis C virus
A hepatitis C virus and target technology, applied in the field of medicine, to achieve the effect of improving the cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
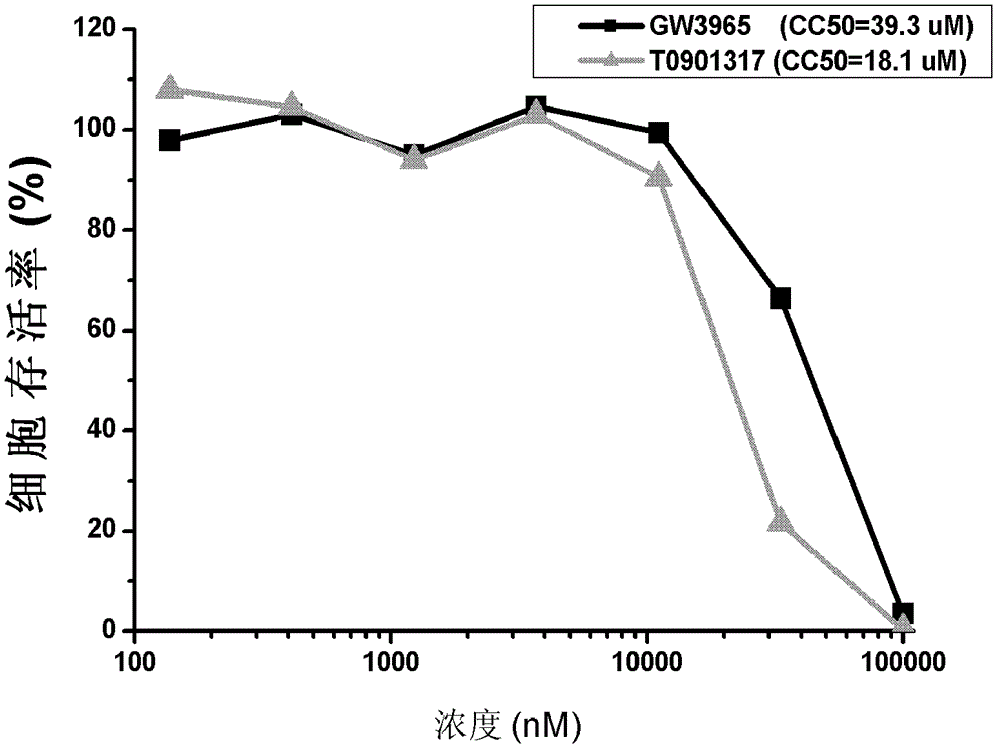
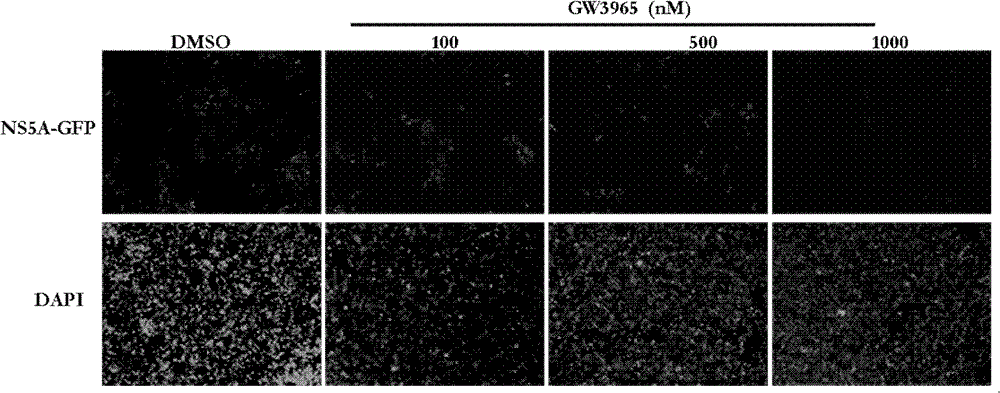
[0041] Evaluation of the anti-hepatitis C virus activity of the LXR agonists GW3965 and T0901317.
[0042] 1. Experimental materials
[0043] 1.1 Cells, plasmids, viruses and drugs
[0044] Huh7.5.1 cells were donated by Dr.F.V.Chisari; plasmid pJFH1 containing the complete genome sequence of HCV type 2a JFH1 strain was donated by Prof. Dr. Takaji Wakita; plasmid pEGFP-N1 was purchased from Clontech; plasmid pGL4.70[hRLuc] was purchased from promega company; the virus JFH1-Luc-5AGFP with double reporter genes was prepared by our laboratory; GW3965 and T0901317 were purchased from sigma company.
[0045] 1.2 Reagents
[0046] DMEM medium was purchased from GIBCO Company; Renilla luciferase detection kit was purchased from Promega Company; anti-HCV NS3 mouse monoclonal antibody was purchased from Henan Bioengineering Technology Research Center; anti-GAPDH mouse monoclonal antibody was purchased from Beijing Zhongshan Golden Bridge Company; HRP-labeled goat anti-mouse secondary ...
Embodiment 2
[0065] Example 2: Study on the Effect of LXR Agonist GW3965 and T0901317GW3965 on the Entry of Hepatitis C Pseudovirus (HCVpp)
[0066] 1. Experimental materials
[0067] 1.1 Cells, viruses and drugs
[0068] Huh7.5.1 cells; GW3965 and T0901317; plasmid PNL4.3-R-E-Luc and plasmid pVpack-VSV-G containing the VSV viral envelope protein were obtained from the AIDS Research Division of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The plasmids containing HCV1a and 1b envelope proteins were donated by Mr. Qi Zhongtian.
[0069] 1.2 Reagents
[0070]DMEM medium was purchased from GIBCO; transfection reagent Lipofectamine2000 was purchased from Invitrogen; Firefly luciferase detection kit was purchased from Promega.
[0071] 1.3 Experimental Instruments
[0072] The 20 / 20 detector is a product of Promega.
[0073] 2. Experimental methods and results
[0074] 2.1 Compared with the live virus, the HCV pseudovirus has similar cell infe...
Embodiment 3
[0076] Example 3: Combination study of LXR agonists GW3965 and T0901317 with other drugs with anti-HCV activity.
[0077] 1. Experimental materials
[0078] 1.1 Cells, viruses and drugs
[0079] Huh7.5.1; virus JFH1-Luc-5AGFP; GW3965 and T0901317; CsA (purchased from Sigma Company) and MK-7009 (purchased from Zannan Company).
[0080] 1.2 Reagents
[0081] DMEM medium was purchased from GIBCO; Renilla luciferase detection kit was purchased from Promega.
[0082] 1.3 Experimental Instruments
[0083] The 20 / 20 detector is a product of Promega.
[0084] 2. Experimental methods and results
[0085] 2.1 Divide Huh7.5.1 cells into 8×10 cells 3 Each cell / well was inoculated in a 96-well cell culture plate, cultured in a 37°C cell culture incubator for 14-18 hours, and then used after the cells grew into a monolayer. Cyclosporine A (CsA) or MK-70092-fold gradient dilution was added to the well plate as a control group for separate medication, and each group had 3 repetitions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com