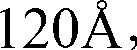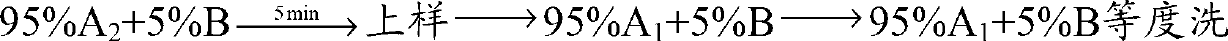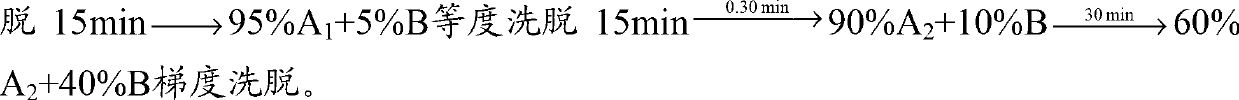Preparation method of desmopressin acetate
A technology of desmopressin and acetic acid, which is applied in the field of preparation of desmopressin acetate, can solve the problems of reduced yield, insoluble crude peptide, and low oxidation efficiency, so as to increase the concentration of oxidized samples and reduce side effects. Reduction of reaction and waste liquid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0057] Example 1: Preparation of Fmoc-Gly-SieberAmide resin
[0058] Add 20g of Sieber Amide resin (substitution degree of 1.2mmol / g) to a 200mL reaction column, add DMF to swell for 30 minutes, after swelling, use 20% hexahydropyridine / DMF(V:V) solution to remove Fmoc protection Group. Wash three times with DMF, then three times with DCM. Dissolve Fmoc-Gly-OH (50mmol) and HOBt (60mmol) with an appropriate amount of DMF. Add DIC (60mmol) under ice water bath, activate for 3 minutes, and add to the above reaction column , The reaction was stirred at room temperature for 120 min. After the reaction, the reaction solution was vacuumed off and washed with DMF three times. Use Kaiser reagent to detect the reaction and block with 20% acetic anhydride or pyridine for 2 hours. The reaction solution was drawn off, washed with DMF three times, and washed three times with DCM. A small amount of resin was shrunk with methanol three times and dried by vacuum. The resin substitution degree ...
Example Embodiment
[0059] Example 2: Preparation of Fmoc-Gly-SieberAmide resin
[0060] Add 100 g of Sieber Amide resin (substitution degree 0.5mmol / g) to a 500mL reaction column, add DMF to swell for 30 minutes, then add N-methylpyrrolidone for 30 minutes, after swelling, use 20% hexahydropyridine DMF The solution removes the Fmoc protecting group. Wash three times with DMF, then three times with DCM. After dissolving Fmoc-Gly-OH (150mmol) and HOBt (200mmol) with an appropriate amount of DMF, add it under ice water bath and activate with DIC (300mmol) for 3 minutes, then add to the above reaction column In, the reaction was stirred at room temperature for 120 min. After the reaction, the reaction solution was vacuumed off and washed with DMF three times. Use Kaiser reagent to detect the reaction and block with 20% acetic anhydride or pyridine for 2 hours. DMF was washed three times, DCM washed three times, a small amount of resin was shrunk with methanol three times, and then vacuumed to drynes...
Example Embodiment
[0061] Example 3: Preparation of linear desmopressin peptide resin
[0062] Add 25g of Fmoc-Gly-Sieber Amide resin prepared in Example 1 (substitution degree is 0.90mmol / g) into a 500mL reaction column, add DMF to swell for 30 minutes, and then use 20% hexahydropyridine DMF solution to remove Fmoc The protecting group is 10 minutes, and then washed three times with DMF and DCM respectively. Dissolve Fmoc-D-Arg(Pbf)-OH (50mmol), HOBt (75mmol), and DIC (100mmol) with an appropriate amount of DMF and add them to the above reaction column. Stir under nitrogen for 120 minutes at room temperature. After the reaction was detected with Kaiser reagent, the reaction solution was vacuumed out, washed with DMF three times, and DCM washed three times. According to the above method of coupling Fmoc-D-Arg(Pbf)-OH, sequentially couple Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt) -OH, Fmoc-Phe-OH, Fmoc-Tyr(tBu)-OH and Mpr(Trt)-OH. Finally, Mpr(Trt)-Tyr(tBu)-Phe-Gln(Trt)-Asn(T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com