A kind of oxide solid electrolyte material and its preparation method and application
A solid electrolyte and oxide technology, applied in circuits, electrical components, secondary batteries, etc., can solve the problems of high price and few sources, and achieve the effects of low price, wide source and high room temperature ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Preparation of calcium-doped lithium lanthanum zirconium-based solid electrolyte material
[0027] The lithium source compound lithium carbonate 7.315 g, the lanthanum source compound lanthanum oxide 11.404 g, the zirconium source compound zirconium oxide 6.161 g, and the calcium source compound calcium carbonate 0.5 g according to the molar ratio of Li, La, Zr, and Ca are 7.92: 2.80: 2: 0.20 , Doping elements accounted for 0.95% of the matrix material, ball milled and mixed in ethanol medium for 10 hours, then dried at 60°C, calcined at 900°C for 15 hours, then ball milled in isopropanol medium for 12 hours, and dried at 75°C After 6 hours, pre-compression molding was carried out at a pressure of 4 MPa for 1 minute, and then cold isostatically pressed at a pressure of 200 MPa for 5 minutes, and the formed body was sintered at 1200°C for 24 hours to obtain the calcium element provided by the present invention Doped with lithium lanthanum zirconium-based solid el...
Embodiment 2
[0029] Example 2. Preparation of strontium-doped lithium lanthanum zirconium-based solid electrolyte material
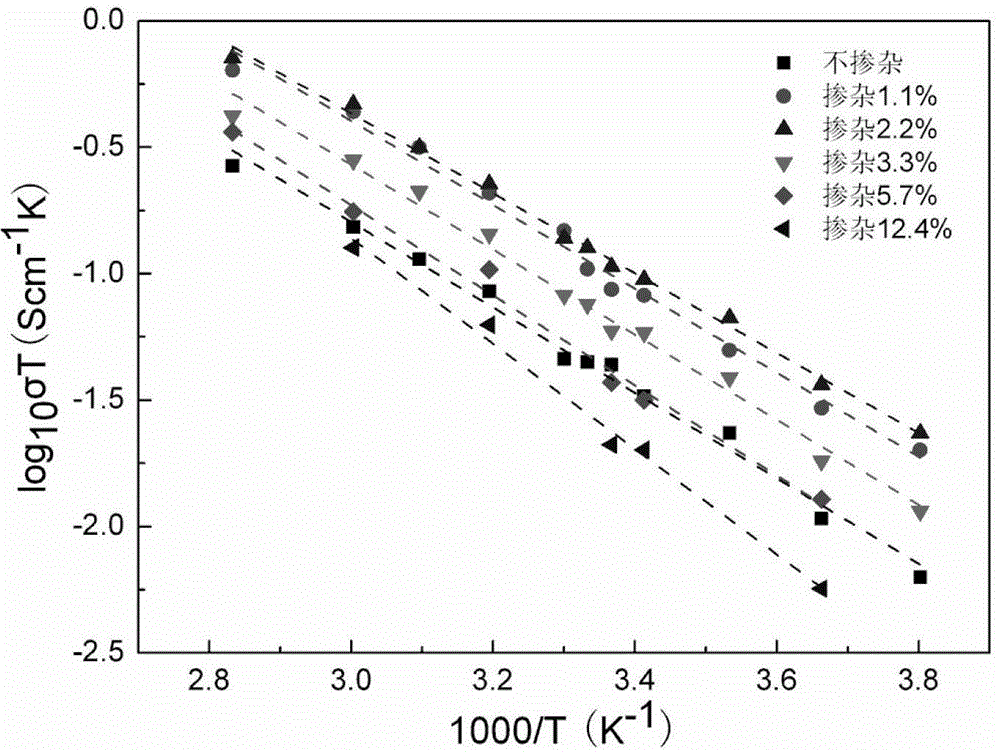
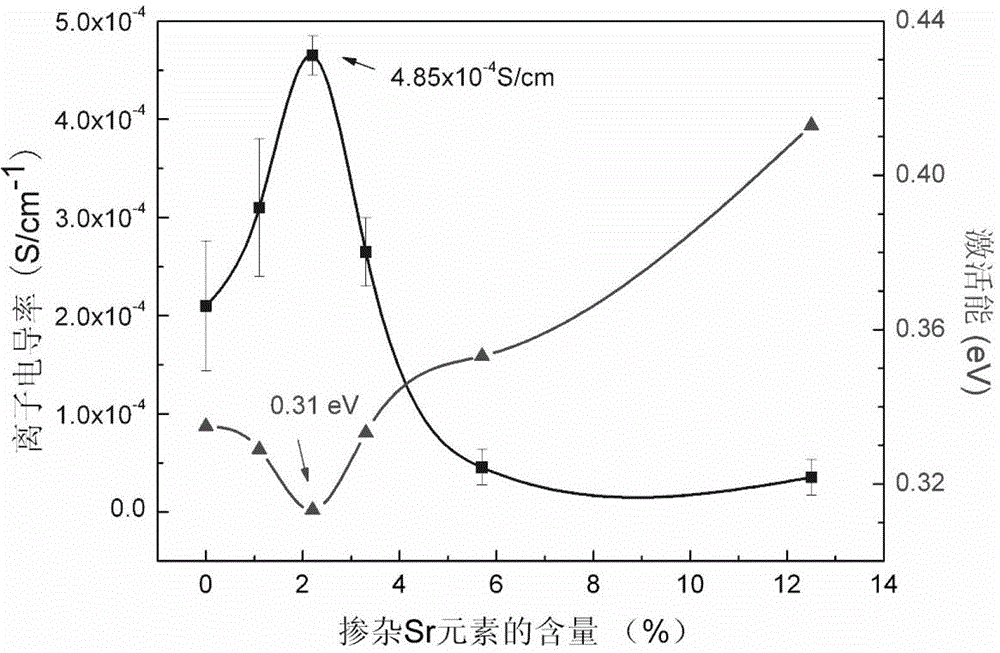
[0030] The lithium source compound lithium carbonate 7.315 g, the lanthanum source compound lanthanum oxide 11.404 g, the zirconium source compound zirconium oxide 6.161 g, and the strontium source compound strontium carbonate 0.738 g according to the molar ratio of Li, La, Zr, Sr are 7.92: 2.80: 2: 0.20 , Doping elements accounted for 2.2% of the matrix material, ball-milled and mixed in ethanol medium for 10 hours, then dried at 60°C, calcined at 900°C for 15 hours, then ball-milled in isopropanol medium for 12 hours, and dried at 75°C After 8 hours, pre-compression molding was carried out at a pressure of 4 MPa for 5 minutes, and then cold isostatically pressed at a pressure of 200 MPa for 5 minutes, and the formed body was sintered at 1200°C for 24 hours to obtain the strontium element provided by the present invention Doped lithium lanthanum zirconium-based solid e...
Embodiment 3
[0034] Example 3 Preparation of barium-doped lithium lanthanum zirconium-based solid electrolyte material
[0035] The lithium source compound lithium carbonate 7.315 g, the lanthanum source compound lanthanum oxide 11.404 g, the zirconium source compound zirconium oxide 6.161 g, and the calcium source compound barium carbonate 0.987 g according to the molar ratio of Li, La, Zr, and Ba are 7.92: 2.80: 2: 0.20 , Doping elements accounted for 3.27% of the matrix material, ball milling and mixing in ethanol medium for 10 hours, then dried at 60 ℃, calcined at 900 ℃ for 15 hours, and then ball milled in isopropanol medium for 12 hours at 75 ℃ After drying for 8 hours, pre-compacting for 10 minutes at a pressure of 4 MPa, and then cold isostatically pressing at a pressure of 200 MPa for 5 minutes, the formed body is sintered at 1200°C for 24 hours to obtain the barium provided by the present invention Element-doped lithium lanthanum zirconium-based solid electrolyte material.
[0036] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com