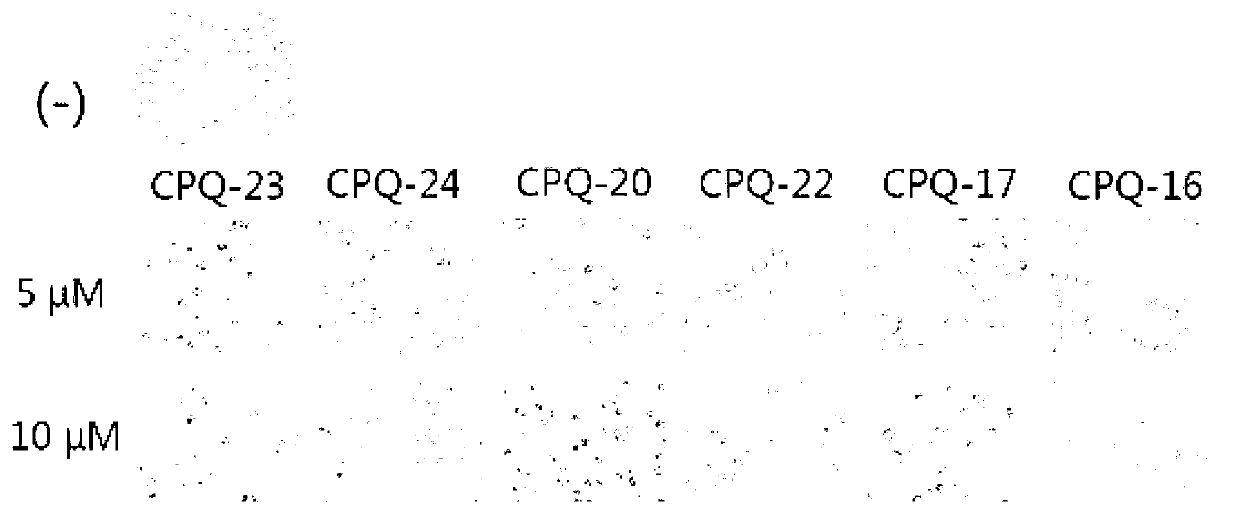
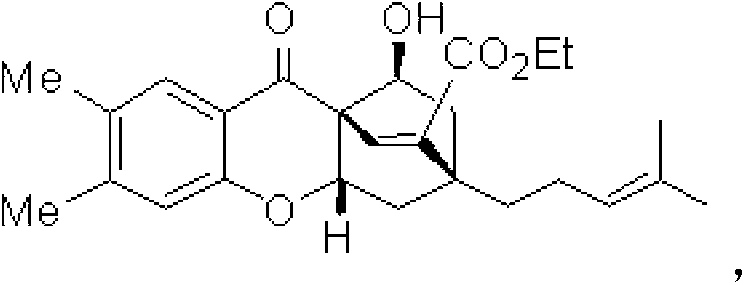
Applications of dihydro chromone framework compounds in preparing medicine for curing malignant tumor
The technology of a skeleton compound, dihydrochromone, is applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc. It can solve the problems of not revealing biological activity and not revealing the effect of killing tumor cells with antitumor activity, and achieve great social benefits and economical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of dihydrochromone skeleton compound CPQ-16
[0030] In the reaction tube, add 0.02mmol hexahydropyridine catalyst and 0.1mmol chromone electron-deficient diene compound successively, namely 0.2mmol long-chain alkenal, namely 0.02mmol acidic additive o-fluorobenzoic acid and 1mL solvent 1,4-dioxane were reacted under normal pressure and 25°C under stirring, and the reaction was monitored by TLC. After 12 hours, the reaction was completed. The solvent was recovered under reduced pressure, and the residue was passed through The target product CPQ-16 was obtained by column chromatography separation with a yield of 68%. The structural formula is as follows:
[0031]
[0032] 1 H NMR (400MHz, CDCl 3):δ=8.04(dd,J=8.8Hz,J=6.4Hz,1H),7.19(s,1H),6.82(td,J=8.4Hz,J=2.4Hz,1H),6.67(dd,J =10.0Hz,J=2.4Hz,1H),5.15(s,1H),4.57(s,1H),4.38(dd,J=10.0Hz,J=2.8Hz,1H),4.21(q,J=7.2 Hz,2H),2.27(dd,J=13.6Hz,J=8.0Hz,1H),2.12-2.05(m,1H),1.97-1.94(m,4H),1.70(s,3H),1...
Embodiment 2
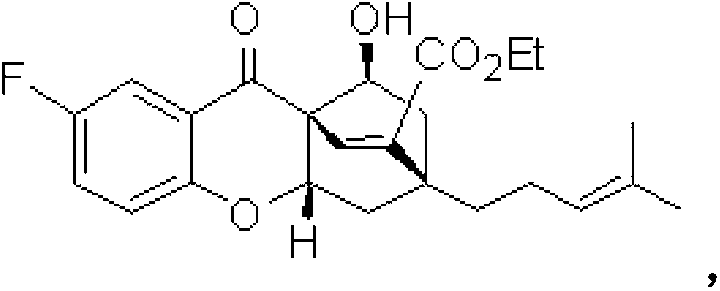
[0033] Embodiment 2: Preparation of dihydrochromone skeleton compound CPQ-17
[0034] In the reaction tube, add 0.02mmol hexahydropyridine catalyst and 0.1mmol chromone electron-deficient diene compound successively, namely 0.2mmol long-chain alkenal, namely 0.02mmol acidic additive o-fluorobenzoic acid and 1mL solvent 1,4-dioxane were reacted under normal pressure and 25°C under stirring, and the reaction was monitored by TLC. After 12 hours, the reaction was completed. The solvent was recovered under reduced pressure, and the residue was passed through The target product CPQ-17 was separated by column chromatography with a yield of 73%. The structural formula is as follows:
[0035]
[0036] 1 H NMR (400MHz, CDCl 3 ):δ=7.74(s,1H),7.20(s,1H),6.76(s,1H),5.16-5.14(m,1H),4.56(dd,J=8.0Hz,J=1.6Hz,1H) ,4.31(dd,J=10.0Hz,J=3.2Hz,1H),4.21(q,J=7.2Hz,2H),2.28(s,3H),2.24-2.22(m,4H),2.09-1.92( m,5H),1.70(s,3H),1.61(s,3H),1.55(dd,J=13.2Hz,J=3.2Hz,1H),1.40(dt,J=13.6Hz,J=2.8Hz, 1H...
Embodiment 3
[0037] Embodiment 3: Preparation of dihydrochromone skeleton compound CPQ-20
[0038] In the reaction tube, add 0.02mmol hexahydropyridine catalyst and 0.1mmol chromone electron-deficient diene compound successively, namely 0.2mmol long-chain alkenal, namely 0.02mmol acidic additive o-fluorobenzoic acid and 1mL solvent 1,4-dioxane were reacted under normal pressure and 25°C under stirring, and the reaction was monitored by TLC. After 12 hours, the reaction was completed. The solvent was recovered under reduced pressure, and the residue was passed through The target product CPQ-20 was obtained by column chromatography separation with a yield of 91%. The structural formula is as follows:
[0039]
[0040] 1 H NMR (400MHz, CDCl 3 ):δ=8.02(dd,J=8.8Hz,J=6.4Hz,1H),7.18(s,1H),6.81(td,J=8.4Hz,J=2.4Hz,1H),6.66(dd,J =9.6Hz,J=2.0Hz,1H),5.16-5.09(m,2H),4.56(s,1H),4.37(dd,J=10.4Hz,J=2.8Hz,1H),4.20(m,2H ),2.30-2.24(m,1H),2.10-1.96(m,9H),1.68(s,3H),1.60-1.54(m,7H),1.43-1.38(m,1H),1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com