Method for in-vitro construction of tissue engineered nerves
A tissue engineering and neural technology, applied in the field of biomedical engineering, can solve different problems and achieve the effect of large surface area
Inactive Publication Date: 2013-08-07
NANTONG UNIVERSITY
View PDF6 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0005] At present, the mechanism of microgravity has not yet been elucidated, and different types of cells may respond quite differently to microgravity, and there is no literature on the attachment of different types of cells to complex structures under microgravity, so different types of cells have been developed. And the study of microgravity three-dimensional culture of complex structural scaffolds to prepare tissue engineered nerves, which has guiding significance for the repair of clinical nerve defects
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
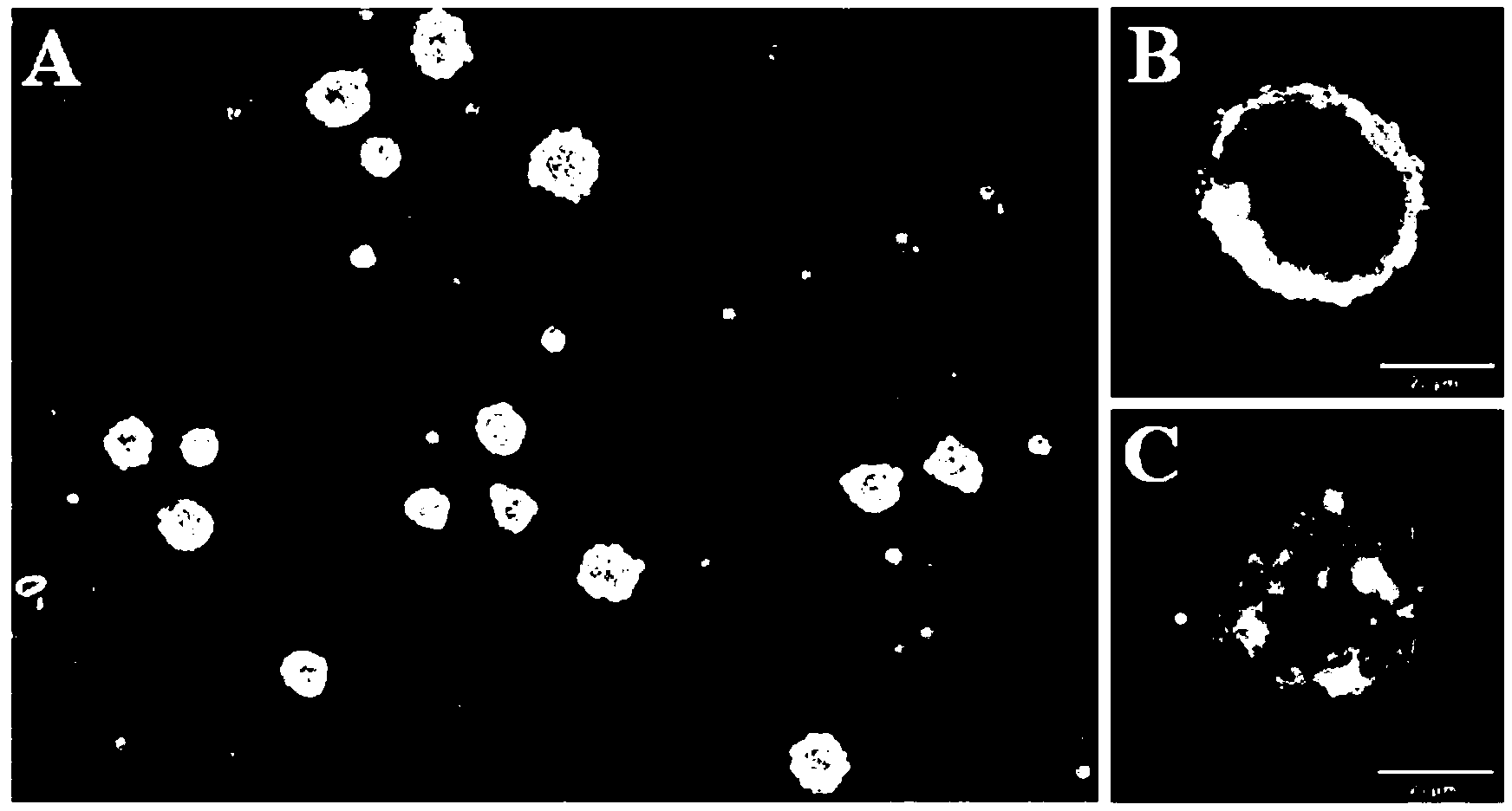
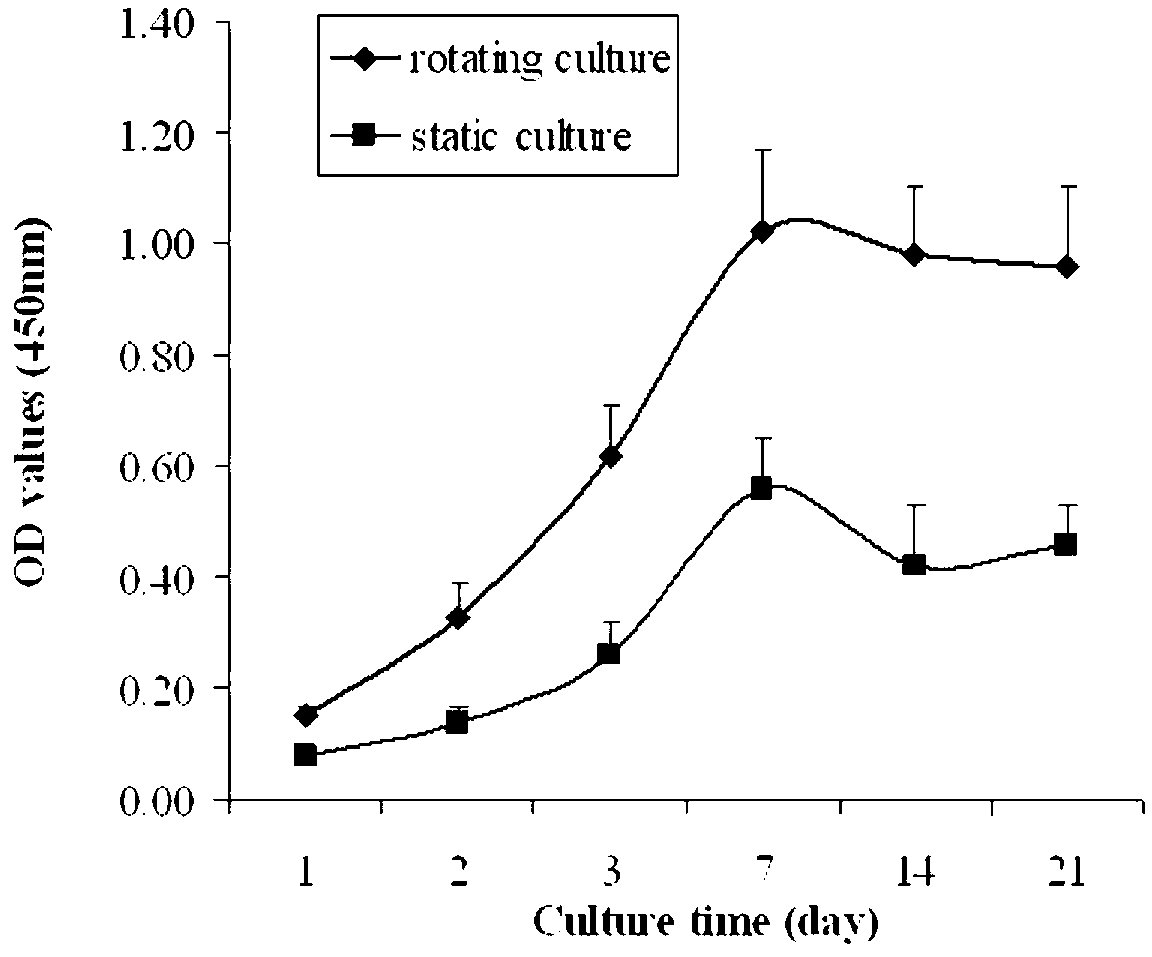
The invention provides a method for in-vitro construction of tissue engineered nerves. The method adopting a three-dimensional gravity bioreactor comprises the following steps of: culturing seed cells with nerve conduits under a three-dimensional micro-gravity environment, wherein the culture conditions are as follows: the seed cell suspension and nerve conduits are placed to a rotary culture container which is filled with complete medium, the cell final density is 1*10<6> / ml, the culture is carried out in a CO2 incubator of 37 DEG C, the rotation speed with the first 24 hours is 10rpm, so that the cells sufficiently contact and are adhered with the nerve conduits; adjusting the rotation speed of the three-dimensional micro-gravity bioreactor after 24 hours, so that the nerve conduits are suspended in the culture solution; and stopping the culture after 7-14 days to obtain the tissue engineered nerves. The method disclosed by the invention can be used for ensuring that the seed cells adhered with the nerve conduits are more and more uniformly, so that peripheral nerve regeneration is facilitated; and moreover, schwann cells, which are induced by skin-orientated precursor cells, are closer to the true schwann cells and are more suitable for being used as seed cells of nerve tissue engineering.
Description
technical field [0001] The invention belongs to the field of biomedical engineering, and specifically relates to a method for constructing tissue-engineered nerves in vitro, in particular to a method for constructing tissue-engineered nerves in vitro by using a three-dimensional microgravity bioreactor. Background technique [0002] Every year in the world, there are many cases of peripheral nerve extrusion, stretching, and contusion injuries due to trauma such as car accidents, sharp objects, and bullets. For short-distance peripheral nerve defects, tension-free surgery can be performed using the elasticity and curvature of the nerve itself. The broken end was sutured to repair the defect. When the defect exceeds a certain distance and cannot be sutured without tension, nerve grafting must be performed. At present, autologous nerve transplantation is the most commonly used clinically, and it is regarded as the gold standard of peripheral nerve repair. However, the sources...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61L27/38A61L27/20A61L27/18A61L27/22
Inventor 顾晓松丁斐薛成斌杨宇民顾芸汤欣朱慧
Owner NANTONG UNIVERSITY
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com