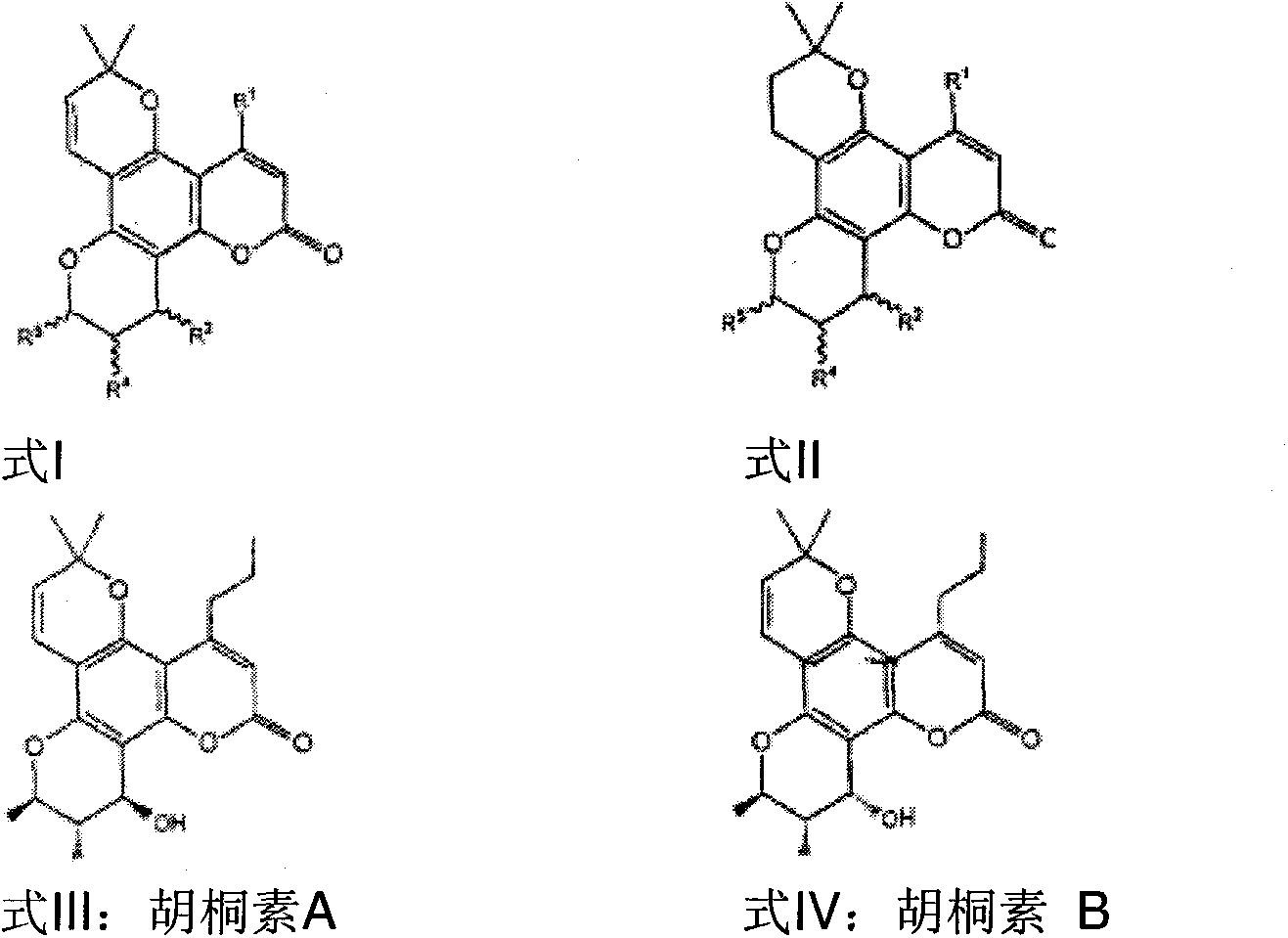
Pharmaceutical compositions for calanolides, their derivatives and analogues, and process for producing the same
A composition, calanolide technology, applied in the directions of drug combination, pharmaceutical formulation, drug delivery, etc., can solve the problem of no report and overcome calanolide, low bioavailability, no disclosure or identification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0242] Preparation of calanoline A solution
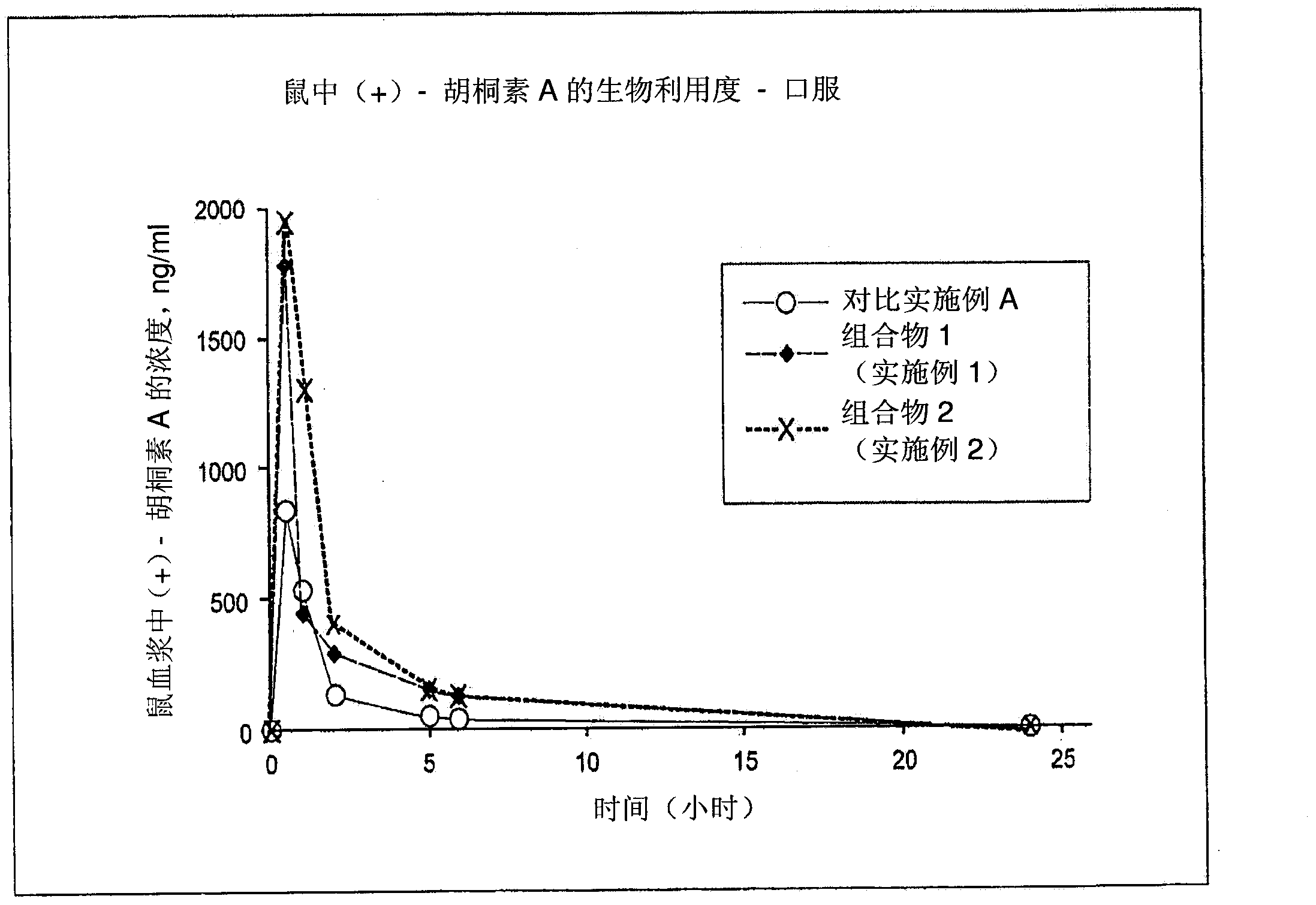
[0243] The following formulation provides a solution of calanoline A suitable for filling soft gels with enhanced water solubility and bioavailability as shown in Examples 9 and 10.
[0244]
[0245] 5.6 kg medium chain triglyceride oil was heated to about 50°C-60°C, then 1.0 kg calanoline A was added and dissolved in the oil. Then PEG-40 hydrogenated castor oil (Cremophor RH 40) was added, the mixture was cooled to room temperature and then 825 mg of the mixture was filled in each soft capsule, each soft capsule contained 100 mg calanoline A for oral administration.
Embodiment 2
[0247] Preparation of calanoline A solution
[0248] The following formulation provides a solution of calanoline A suitable for filling soft gels with enhanced water solubility and bioavailability as shown in Examples 9 and 10. .
[0249]
[0250] 1.0 kg of medium chain triglyceride oil and 2.0 kg of polyethylene glycol 200 were heated to about 50°C-60°C, then 1.0 kg of calatonin A was added and dissolved in the mixture. Then 3.8 kg polysorbate 80 and 0.2 kg sorbitan monolaurate were added and the mixture was cooled to room temperature. Then fill 800mg mixture in each soft capsule, then contain 100mg calanoline A in each soft capsule for oral administration.
Embodiment 3
[0252] Preparation of calanoline A solution
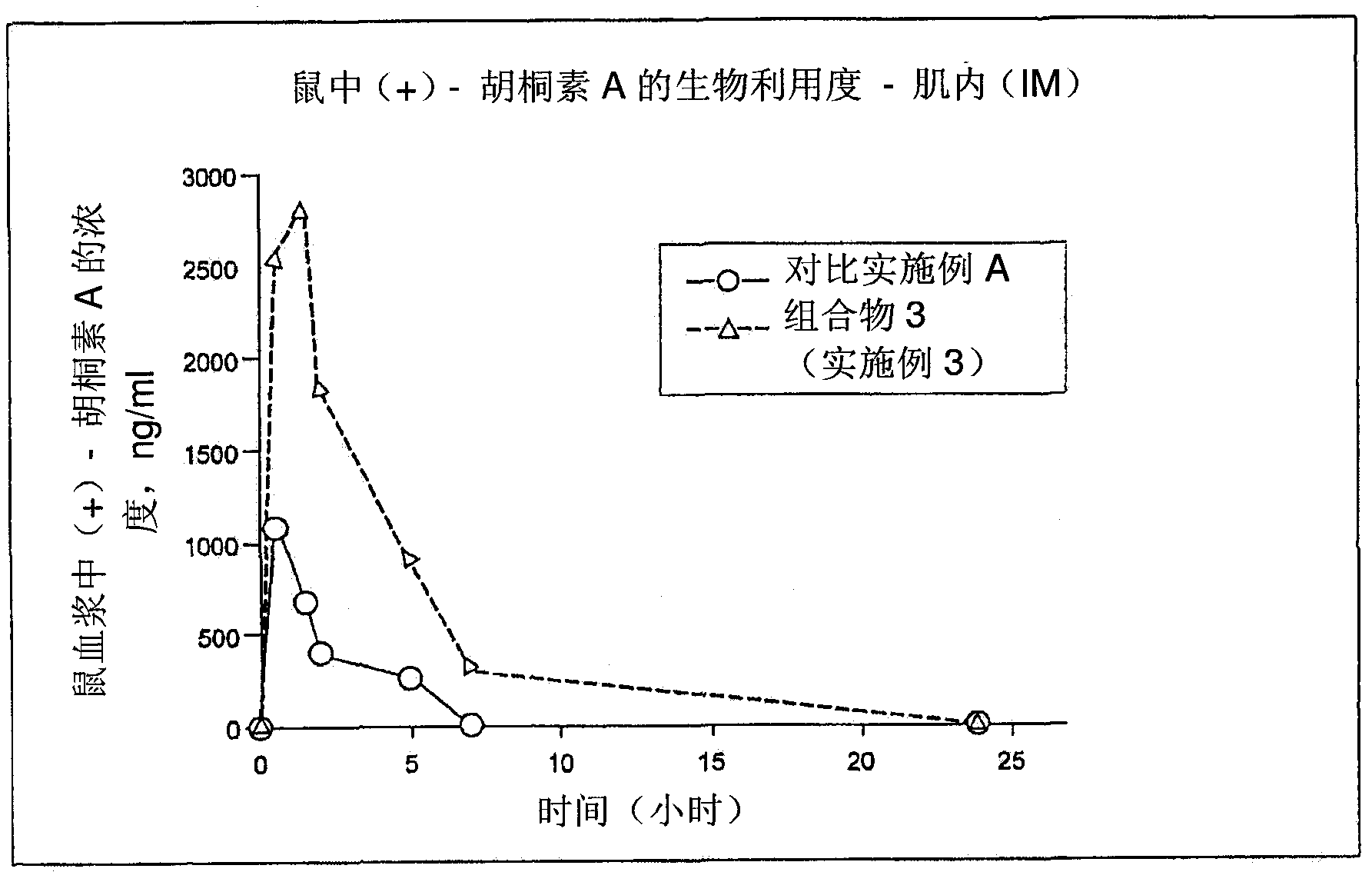
[0253] The following formulation provides a solution of calanoline A suitable for aseptic filling for injection (intramuscular or subcutaneous) with enhanced water solubility and bioavailability as shown in Examples 9 and 11.
[0254]
[0255]
[0256] At 20°C-30°C, 1.0 kg calanolide A was dissolved in 6.32 kg N-methylpyrrolidone. Then 0.4 kg polysorbate 20 and 0.28 kg PEG-40 hydrogenated castor oil were added with stirring until a homogeneous solution was formed and 800 mg of the solution was aseptically filled in each vial for injection.
[0257] Each vial contains 100 mg calanoline A for intramuscular or subcutaneous administration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com