Novel combinations
A technology for active ingredients, pharmaceutical products, applied in new combination fields, which can solve problems such as unsatisfactory efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0309] Methoxyacetic acid (1R,2R,3aS,3bS,10aS,10bR,11S,12aS)-10b-fluoro-1-{[(fluoromethyl)thio]carbonyl}-7-(6-fluoropyridine-3 -yl)-11-hydroxyl-2,10a,12a-trimethyl-1,2,3,3a,3b,4,5,7,10,10a,10b,11,12,12a-tetrahydrocyclo Penta[5,6]naphtho[1,2-f]indazol-1-ester (Compound A)
[0310]
[0311] In a 250 mL round bottom flask Intermediate 5 (8.8 g, 17.07 mmol) was dissolved in DCM (80 mL) and triethylamine (5.91 mL, 42.67 mmol) was added. To the stirred mixture was added 2-methoxyacetyl chloride (3.89 g, 35.84 mmol) while cooling in a water bath, and the mixture was stirred for 10 minutes. Add N 1 -Ethyl-N 2 ,N 2 - Dimethylethane-1,2-diamine (3.48 mL, 22.19 mmol) and the mixture was stirred for another 10 minutes. A 60% solution of bromofluoromethane (4.82 g, 25.60 mmol) in DMF was added followed by triethylamine (2 ml) and the reaction mixture was stirred for a further 30 minutes. The resulting mixture was concentrated in vacuo and partitioned between EtOAc (150ml) and 1M H...
Embodiment 2
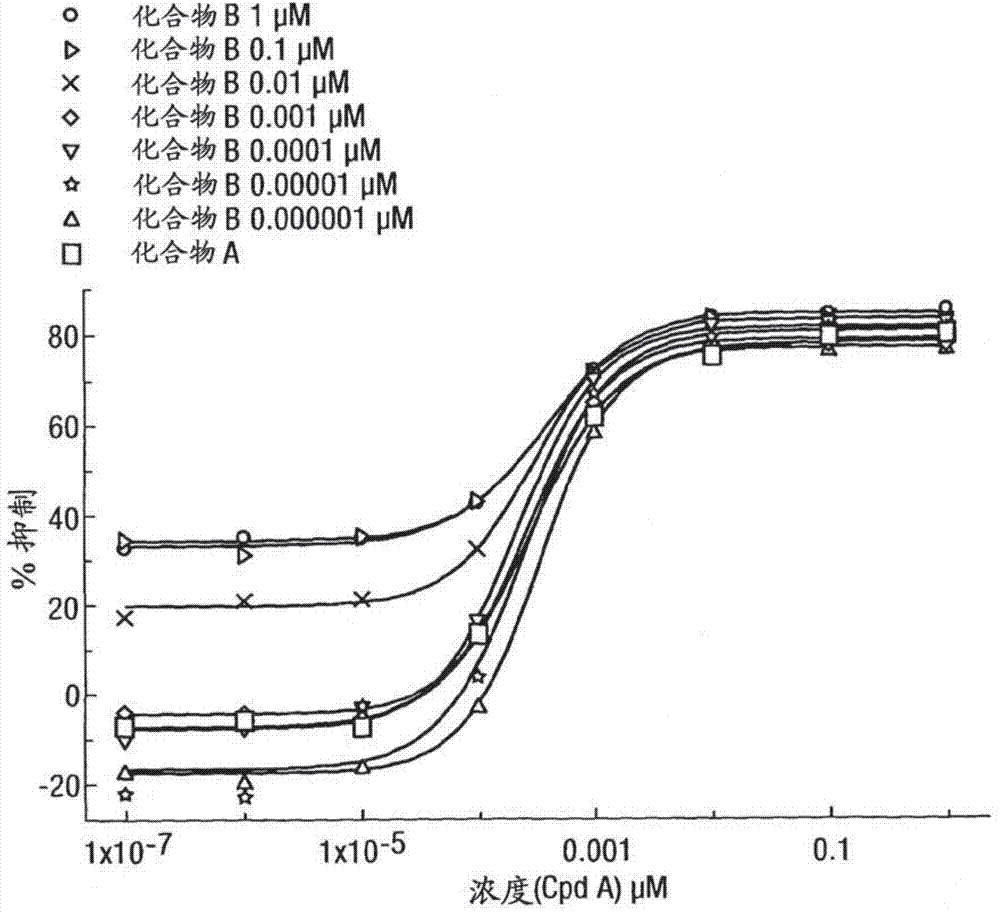
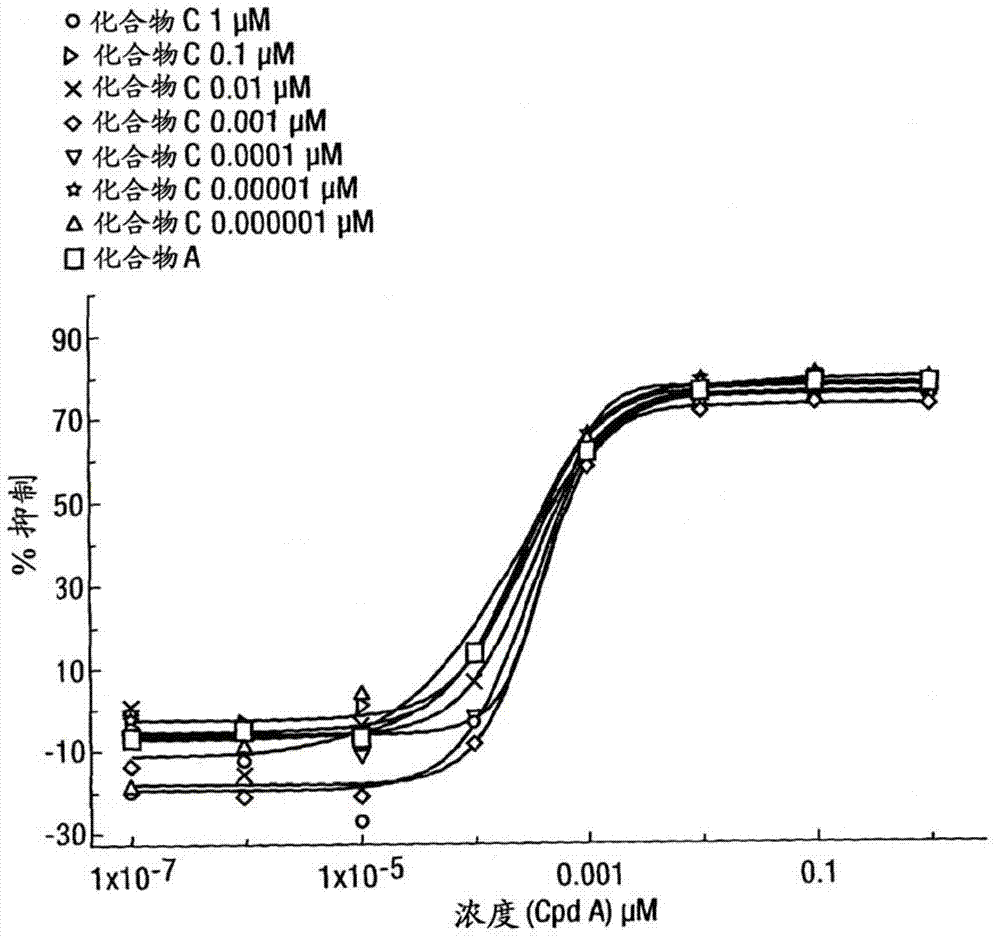
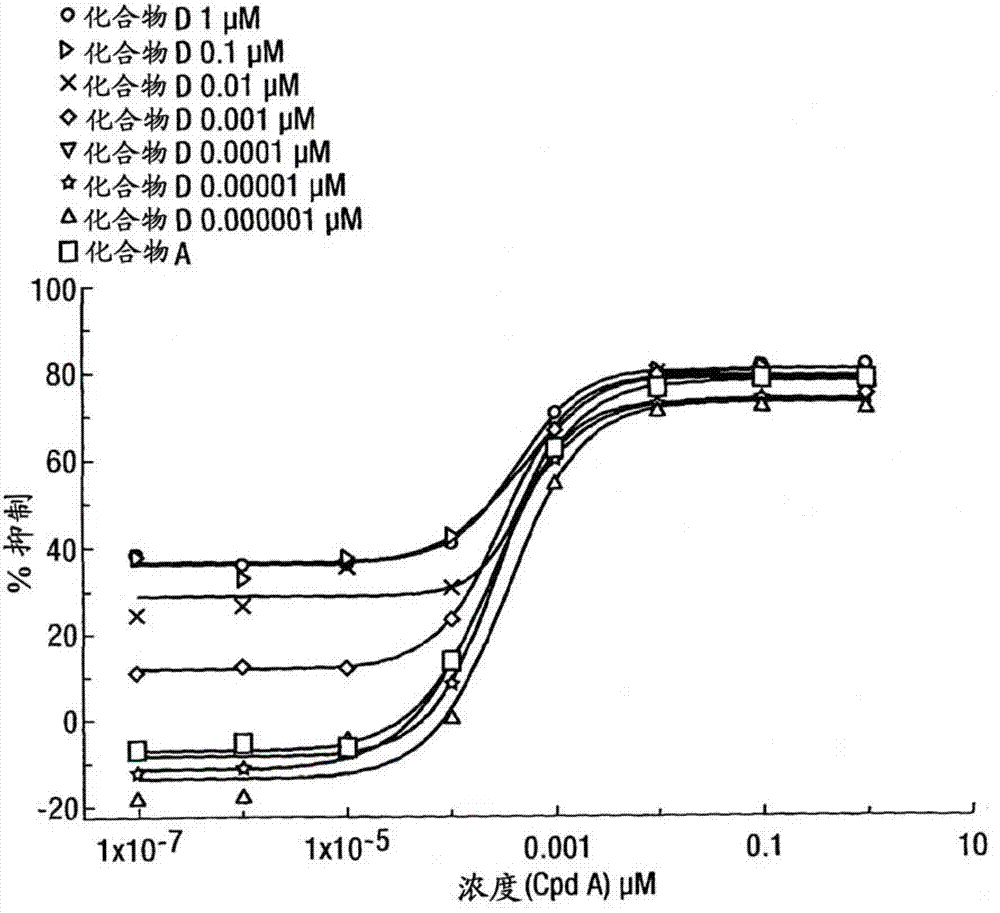
[0316] Inhibition of lipopolysaccharide (LPS)-induced TNFα production in human peripheral blood mononuclear cells
[0317] Peripheral blood mononuclear cells (PBMC) isolated from humans were treated with a range of concentrations of the GR agonist methoxyacetic acid (1R, 2R, 3aS, 3bS, 10aS, 10bR, 11S, 12aS)-10b-fluoro-1-{[( Fluoromethyl)thio]carbonyl}-7-(6-fluoropyridin-3-yl)-11-hydroxy-2,10a,12a-trimethyl-1,2,3,3a,3b,4,5 ,7,10,10a,10b,11,12,12a-tetrahydrocyclopenta[5,6]naphtho[1,2-f]indazole-1-ester (compound A) (alone or in a In the presence of a series of concentrations of a second compound with different pharmacological activity) pre-incubated at 37°C for 45 minutes. The second compound is selected from β 2 Adrenoceptor agonists, double beta 2 Adrenergic receptor agonists / M3 receptor antagonists (hereinafter referred to as "MABA compounds"), muscarinic antagonists or p38 kinase inhibitors. After a pre-incubation period, cells were then incubated with LPS (5 ng / mL) for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com