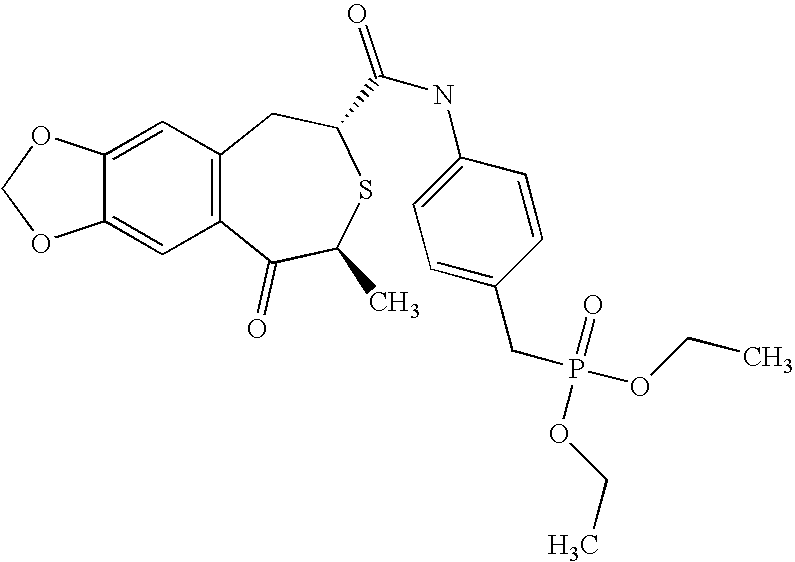
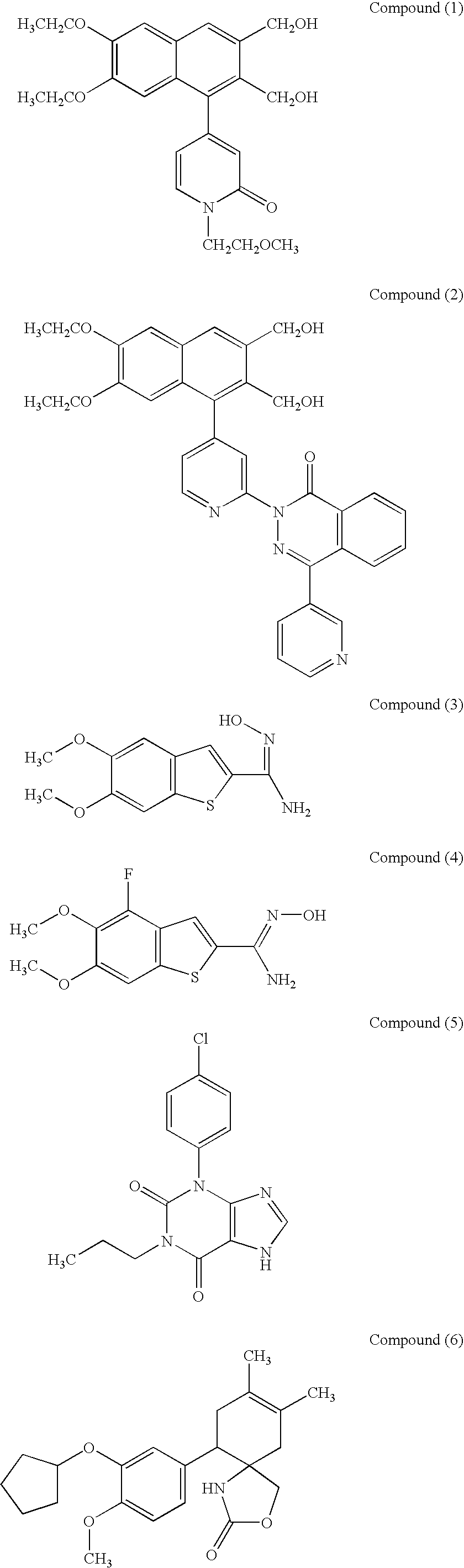
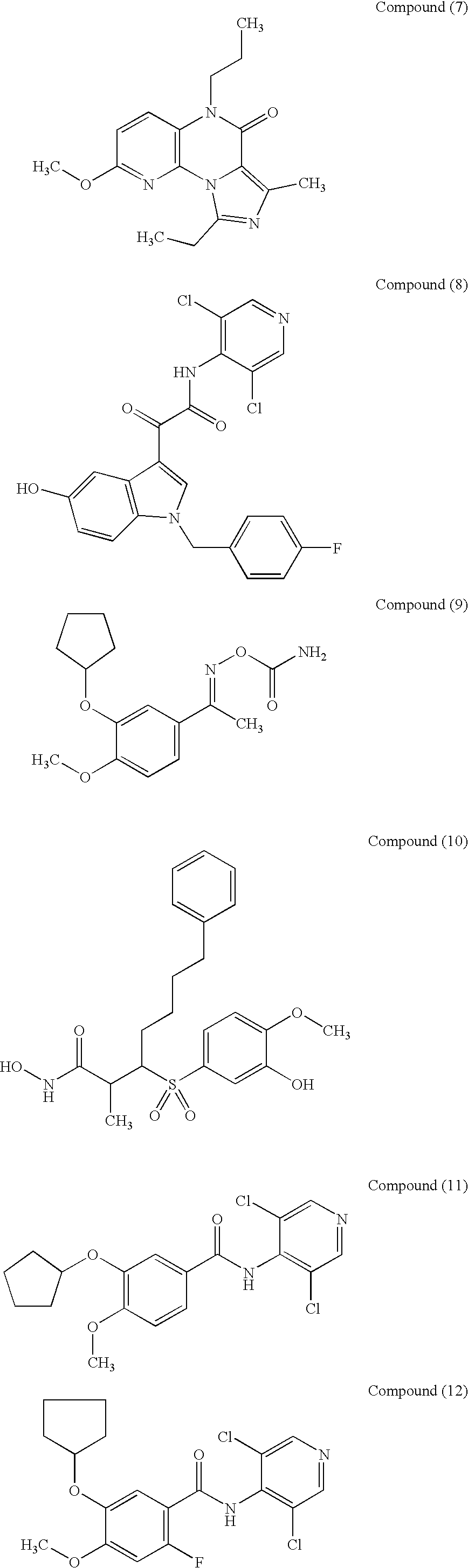
Compositions for promoting healing of bone fracture
a technology for fracture healing and compositions, applied in the direction of drug compositions, prosthesis, transportation and packaging, etc., can solve the problems of limiting the patient's daily life, and affecting the healing effect of bone fractures, so as to accelerate the healing of bone fractures and accelerate the healing of fractures. , the effect of efficient fracture healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
Acceleration of Fracture Healing in Normal Rat
[0108] (Acclimation)
[0109] CD (SD) IGS rats (Charles River Japan, Inc.; male; 7-week-old) were housed for seven days at room temperature (23.+-.2.degree. C.) and 40-70% humidity. During the housing period, the rats were free to access commercially available food (from Oriental Bio; CE-2).
[0110] (Fracture Healing)
[0111] Under ether anesthesia, the left-lower legs of rats were shaved and sterilized with 70% aqueous ethanol, fibulas were exposed with scissors and cut with nail scissors (Natsume Seisakusyo; B17). The cut sections of the fibula were re-matched with tweezers. For the test groups (20 rats / group), the drug-containing microspheres prepared in Example 2-(1), which contains 0.1 or 0.5 mg of Compound (1), were placed around the cutting site of each rat using spatula followed by suturing with silk thread. For the control group (20 rats / group), the same amount of drug-free microspheres prepared in Control Example 1-(1) were placed aro...
experimental example 2
Acceleration of Fracture Healing in Normal Rats
[0127] (Acclimation)
[0128] CD (SD) IGS rats (Charles River Japan, Inc.; male; 7-week-old) were housed for seven days at room temperature (23+2.degree. C.) and 50.+-.20% humidity. During the housing period, the rats were free to access commercially available food (from Oriental Bio; CE-2).
[0129] (Fracture Healing)
[0130] Under ether anesthesia, the left-lower legs of rats were shaved and sterilized with 70% aqueous ethanol; fibulas were exposed with scissors and cut with nail scissors (Natsume Seisakusyo; B17). The cut sections of the fibula were re-matched with tweezers. For the test groups (15 rats / group), the drug-containing microspheres prepared in Example 7, which contains 0.004, 0.02, 0.1 or 0.5 mg of Compound (2), were placed around the cutting site using spatula followed by suturing with silk thread. For the control group (15 rats / group), the same amount of drug-free microspheres prepared in Control Example 2 were placed around th...
experimental example 3
In Vitro Test
[0144] (Isolation of Costicartilage Cell)
[0145] Costicartilages were isolated from NZ line rabbit (Kitayama Labes., Co Ltd.; male; 4-week-old) and soaked into Hank's balanced salt solution (calcium- and magnesium-free; LifeTech Co., Ltd.; hereinafter, referred to as HBSS"). One costicartilage and one costa were excised together, the adipose tissue and the muscle tissue were removed and then, the proliferating chondrocyte layer of costicartilage was excised. The collected proliferating chondrocyte layer was cut into sections with a surgical knife (FEATHER Safety Razor Co., Ltd.) and the all proliferating chondrocyte layer sections from 4 rabbits were combined in a centrifuge tube. To the centrifuge tube was added 40 ml of HBSS (pH 7.2) supplied with 0.1% tetrasodium ethylenediamine tetraacetate to obtain suspension of the proliferating chondrocyte layer sections, which was shaken at 37.degree. C. for 20 minutes and centrifuged (1500 rpm, 10 minutes). The supernatant was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com