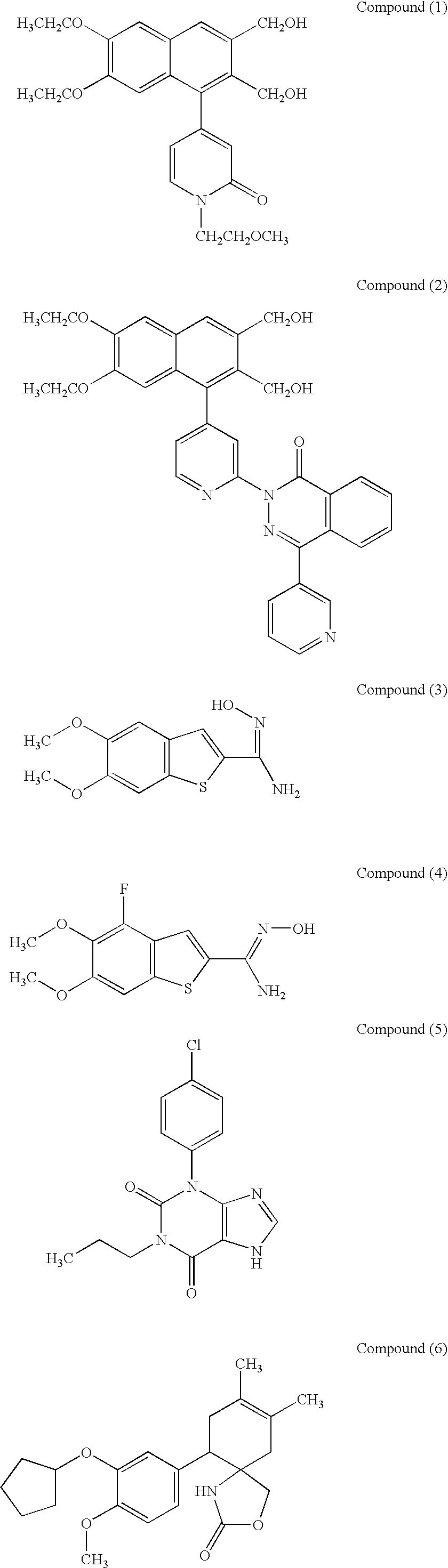
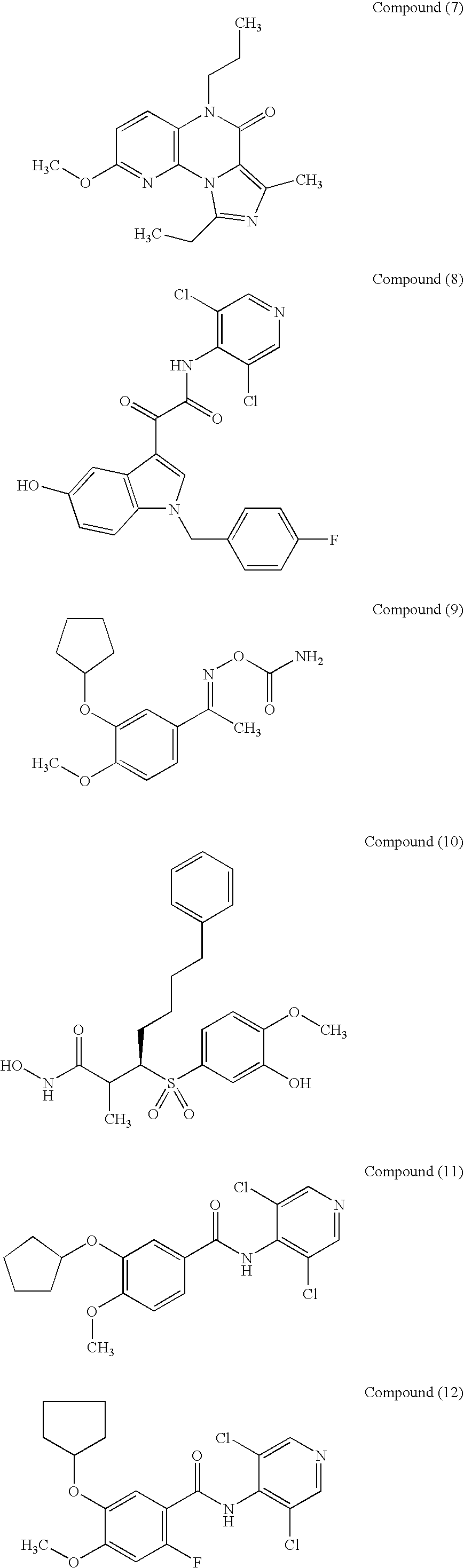
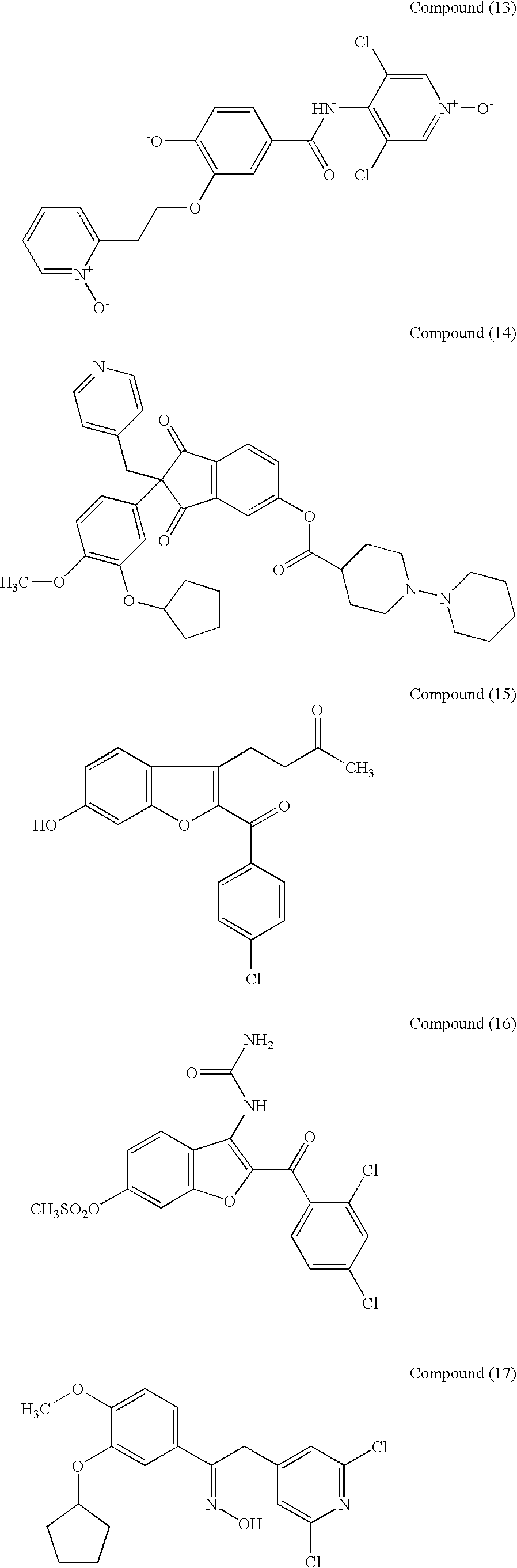
Therapeutic composition for repairing chondropathy
a technology for chondropathy and therapeutic compositions, applied in the direction of drug compositions, biocide, heterocyclic compound active ingredients, etc., can solve the problems of inability to regenerate chondropathy, extremely low regenerative ability of cartilage, etc., and achieve the effect of reducing irritation at the affection si
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example 1
Increase of Matrix Production of Articular Chondrocytes
[0111]
1 Test Compounds Compound(1) (10.sup.-5 M or 10.sup.-4 M); Compound(2) (10.sup.-6 M or 10.sup.-5 M); Compound(9) (10.sup.-6 M or 10.sup.-5 M); Compound(11) (10.sup.-6 M); Compound(21) (10.sup.-6 M or 10.sup.-5 M); Compound(27) (10.sup.-6 M or 10.sup.-5 M); Compound(44) (10.sup.-5 M or 10.sup.-4 M);
[0112] (Isolation and Maintenance of Articular Chondrocytes)
[0113] Four NZW line rabbits (Kitayama Labes., Co Ltd.; male; 4-week-old) were sacrificed with bleeding under ether anesthesia and femur knee joints were collected aseptically. The collected knee joints were stored in phosphate buffer (pH 7.2) containing 0.2% glucose and only the cortical layer of knee joint was scrapped with a surgical knife into a 50 ml tube containing phosphate buffer (pH 7.2) containing 0.2% glucose. The collected knee joint cortical layer was cut into as small sections as possible on a dish with a razor and shaken at 37.degree. C. for 15 minutes in ...
experimental example 2
Fractionation of cAMP-Hydrolyzing PDE Expressed in Articular Cartilage
[0117] Four NZW line rabbits (Kitayama Labes., Co Ltd.; male; 4-week-old) were sacrificed with bleeding under ether anesthesia and femur knee joints were collected aseptically. 15 The collected knee joints were stored in phosphate buffer (pH 7.2) containing 0.2% glucose and only the cortical layer of knee joint portion was scrapped with a knife into a 50 ml tube containing phosphate buffer (pH 7.2) containing 0.2% glucose. The collected knee joint cortical layer was cut into as small sections as possible on a dish with a razor, washed with ice-cold phosphate buffer and homogenized with a homogenizer (Polytron: Kinematica A.G.) in homogenization buffer (20 mM Tris-HCl, pH 7.4, 2 mM magnesium acetate, 0.3 mM calcium chloride, 1 mM dithiothreitol, 40 .mu.M leupeptin, 1.3 mM benzamidine, 0.2 mM phenylmethylsulfonyl fluoride and 1 mM sodium azide). The resultant homogenate was centrifuged (100,000.times.g, 60 minutes) ...
experimental example 3
Expression of A Gene Encoding Cartilage Matrix Protein
[0121] Total RNA was extracted with ISOGEN (Nippon Gene Co., Ltd.) from rabbit knee articular chondrocytes cultured for 4 days according to the same manner as Experimental Example 1 in the presence of Compound (1). at a final concentration of 1.times.10.sup.-4 M or 1.times.10.sup.-5 M, and 15 .mu.g of the total RNA was dissolved in 4.5 .mu.l of a sterilized water. This solution was combined with 2 .mu.l of 5.times.MOPS buffer, 3.5 .mu.l of formaldehyde and 10 .mu.l of formamide, and denatured at 90.degree. C. for 15 minutes. The mixture was then electrophoresed on 1% agarose gel in the presence of formaldehyde. After completion of electrophoresis, RNA was transferred to a nylon membrane (Amersham Pharmacia Biotech) overnight by capillary method. The RNA was fixed to the nylon membrane by UV crosslinking and subjected to prehybridization at 60.degree. C. for 2 hours in 50 ml of hybridization solution (6.times.SSC, 5.times.Denhart'...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com