Pharmaceutical composition for preventing or treating cold injury and thrombus
A composition and drug technology, applied in the field of medicine, to achieve the effect of shortening the length of thrombus formation, reducing drug side effects, preventing or treating cold injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
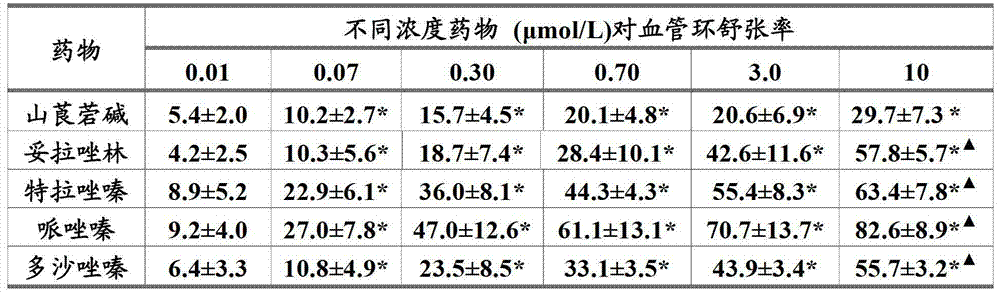
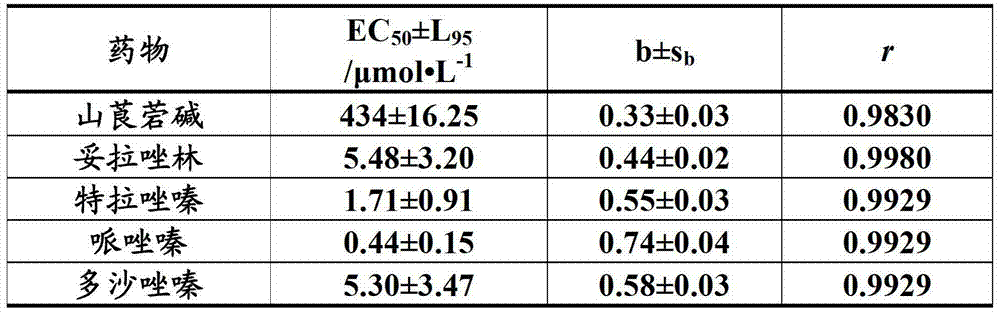
[0033] Example 1 Effects of anisodamine, prazosin and their compositions on the length of tail thrombus in mice induced by carrageenan in cold environment.
[0034] Experimental method: SPF grade male Kunming mice weighing 18-22 g (purchased from the Experimental Animal Center of the Academy of Military Medical Sciences) were given 5 mg.kg of type I carrageenan -1 (0.05% solution prepared with normal saline, intraperitoneally injected at a volume of 0.1ml per 10g of body weight), the mice in the test drug group were given the test drug by intragastric prophylaxis at 0.1ml / 10g body weight 30 minutes before injection of carrageenan (also prepared with normal saline) (concentrations of anisodamine are 0.008%, 0.024%, 0.072%, prazosin concentrations are 0.0007%, 0.002%, 0.006% (concentration unit g / ml) The two drugs were combined and prepared in normal saline. The model group was given normal saline). After injecting carrageenan, the mice were exposed to cold at 18±1°C and 30-50%...
Embodiment 2
[0040] Example 2 The effect of the composition of anisodamine and prazosin on the length of tail thrombus induced by carrageenan in mice under cold environment.
[0041] Experimental model: SPF-grade male Kunming mice weighing 18-22 g were given 2.5 mg.kg of type I carrageenan -1 (0.025% solution prepared with normal saline, injected intraperitoneally at a volume of 0.1ml per 10g of body weight), the mice in the test drug group were given the test drug by intragastric prophylaxis at 0.1ml / 10g body weight 30 minutes before injection of carrageenan (prepared with normal saline) (in each dose group, the concentrations of anisodamine were 0.025%, 0.02%, 0.016%, 0.012%, 0.008%, and the concentrations of prazosin were 0.275%, 0.021%, 0.002%, 0.006% (concentration unit g / ml), the blank control group was intraperitoneally injected and gavaged with the same volume of normal saline, the model group was only given type Ⅰ carrageenan, and no test drug was given, and then the mice were rea...
Embodiment 3
[0048] Example 3 Effects of anisodamine combined with prazosin and tolazoline on blood coagulation and fibrinolysis in mice with carrageenan-induced thrombosis in cold environment.
[0049] The modeling and experimental methods of this example are the same as those of Example 2, and the content of plasma prothrombin time (PT), activated partial thromboplastin time (APTT), and plasma tissue plasminogen activator (t-PA) is detected and plasminogen activator inhibitor-1 (PAI-1) levels. (Use the platelet aggregation coagulation factor analyzer for further operation according to the requirements of the PT and APTT assay kit instructions; for the determination of t-PA and PAI-1 content, follow the requirements of the enzyme-linked immunosorbent assay kit instructions).
[0050] Experimental results: Compared with the blank control group, the PT of the model group was significantly shortened, the plasma t-PA content was significantly reduced, and the PAI-1 content was significantly i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com