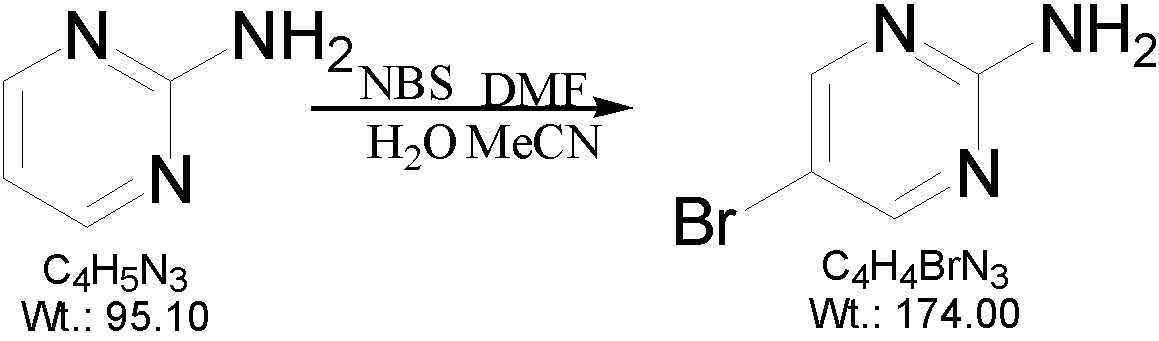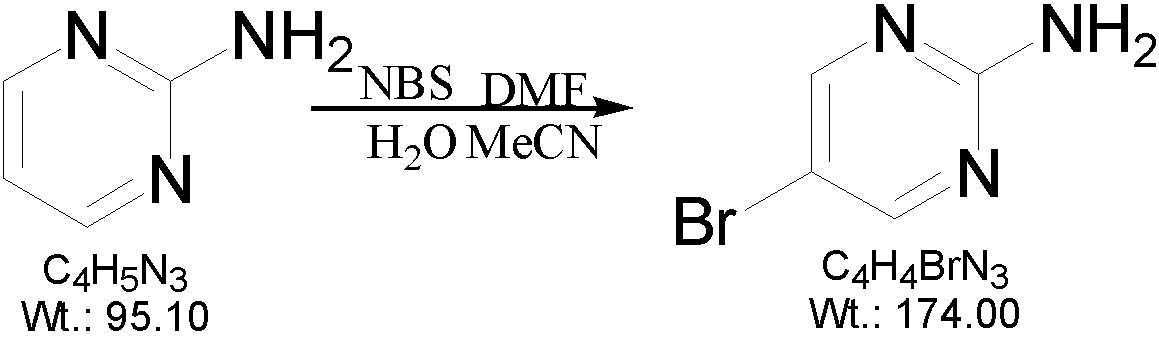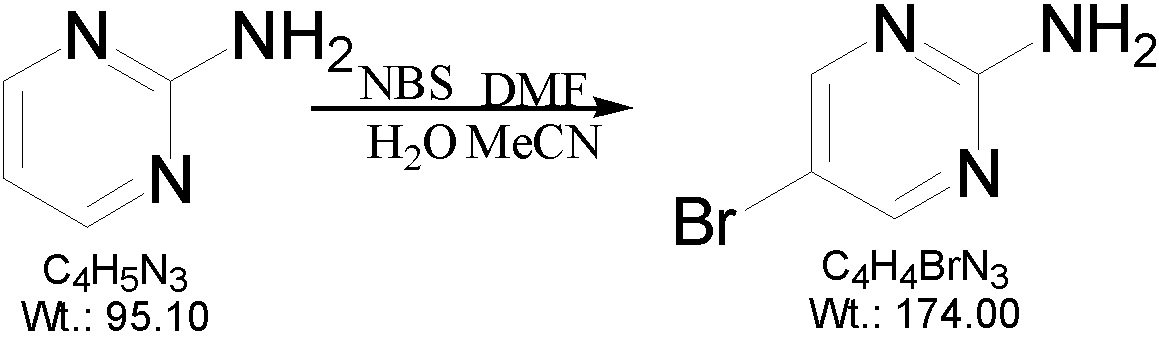Preparation method of 2-azyl-5-bromopyrimidine
A technology of aminopyrimidine and bromopyrimidine, which is applied in the field of preparation of 2-amino-5-bromopyrimidine, can solve the problems of complicated operation, polluted environment, expensive raw materials, etc., and achieves the effects of high chemical yield, convenient purification and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A preparation method of 2-amino-5-bromopyrimidine, which is characterized in that 2-aminopyrimidine and N-bromosuccinimide are used as raw materials, soft water and acetonitrile are used as solvents, and finally prepared by bromination reaction 2-amino-5-bromopyrimidine, the reaction formula is as follows:
[0020]
[0021] The preparation method of a kind of 2-amino-5-bromopyrimidine is characterized in that it specifically comprises the following steps:
[0022] (1) Add 300ml of soft water and 50ml of acetonitrile to a 500ml three-necked reaction flask, and add 15g of 2-aminopyrimidine under stirring;
[0023] (2) Turn on the heating device of the three-necked reaction flask, raise the temperature to 70°C, and stir for 15 minutes.
[0024] (3) Inject 5ml of N-dimethylformamide with a disposable syringe.
[0025] (4) Slowly add 35 grams of N-bromosuccinimide into the three-necked reaction flask three times under stirring. A large amount of white solids precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com