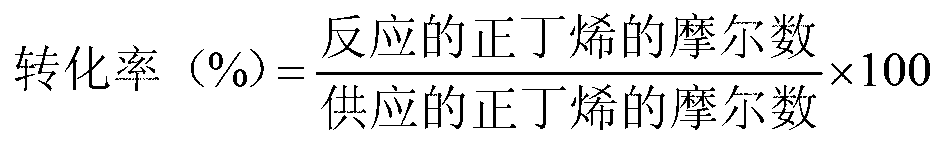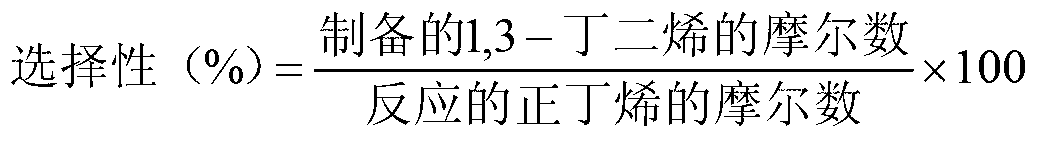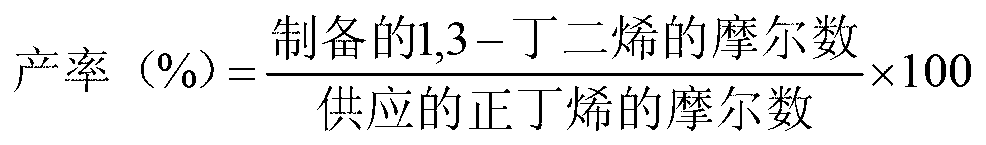High yield production method for 1,3-butadiene
一种丁二烯、高产率的技术,应用在高产率制备1,3-丁二烯领域,能够解决2-丁烯低活性、丁烯异构体活性不同、1-丁烯低活性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0052]
[0053] cesium nitrate (CsNO 3 ) as a cesium precursor, cobalt nitrate hexahydrate (Co(NO 3)2 ·6H 2 O) used as cobalt precursor, iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) used as iron precursor, bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) was used as a bismuth precursor, and ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) were used as molybdenum precursors. Fully dissolve other metal precursors in distilled water, and fully dissolve bismuth nitrate pentahydrate in strong acid solution. Therefore, bismuth nitrate pentahydrate was dissolved separately by adding nitric acid solution to distilled water.
[0054] To prepare the first catalyst, the molar ratio of molybdenum:bismuth:iron:cobalt:cesium was set to 12:1:2:7:0.6.
[0055] 18.0 g of cesium nitrate hydrate (CsNO 3 ), 320.8g cobalt nitrate hexahydrate (Co(NO 3)2 ·6H 2 O) and 125.9g iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) Dissolve in 250 mL of distilled water, then...
preparation Embodiment 2
[0060]
[0061] Potassium nitrate (KNO 3 ) used as potassium precursor, cesium nitrate (CsNO 3 ) as a cesium precursor, cobalt nitrate hexahydrate (Co(NO 3)2 ·6H 2 O) used as cobalt precursor, iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) used as iron precursor, bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) was used as a bismuth precursor, and ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) were used as molybdenum precursors. And the bismuth nitrate pentahydrate is fully dissolved in the strong acid solution. Therefore, bismuth nitrate pentahydrate was dissolved separately by adding nitric acid solution to distilled water.
[0062] To prepare the second catalyst, the molar ratio of molybdenum: bismuth: iron: cobalt: cesium: potassium was set to 12:1:1:7:0.15:0.06.
[0063] With 105.9g bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) Add to the solution of 33.0g nitric acid in 28mL distilled water, then stir to dissolve. After confirmin...
preparation Embodiment 3
[0068]
[0069] Potassium nitrate (KNO 3 ) used as potassium precursor, cesium nitrate (CsNO 3 ) as a cesium precursor, cobalt nitrate hexahydrate (Co(NO 3)2 ·6H 2 O) used as cobalt precursor, iron nitrate nonahydrate (Fe(NO 3 ) 3 9H 2 O) used as iron precursor, bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) was used as a bismuth precursor, and ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) were used as molybdenum precursors.
[0070] To prepare the second catalyst, the molar ratio of molybdenum: bismuth: iron: cobalt: cesium: potassium was set to 12:1:1:7:0.07:0.06.
[0071] With 105.9g bismuth nitrate pentahydrate (Bi(NO 3 ) 2 ·5H 2 O) Add to acid in 28 mL of distilled water to which 33.0 g of nitric acid has been added, and then stir to dissolve. After confirming that the bismuth had been completely dissolved, hydrated cesium nitrate (CsNO 3 ), 1.3g potassium nitrate (KNO 3 ), 449.3g cobalt nitrate hexahydrate (Co(NO 3)2 ·6H 2 O) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com