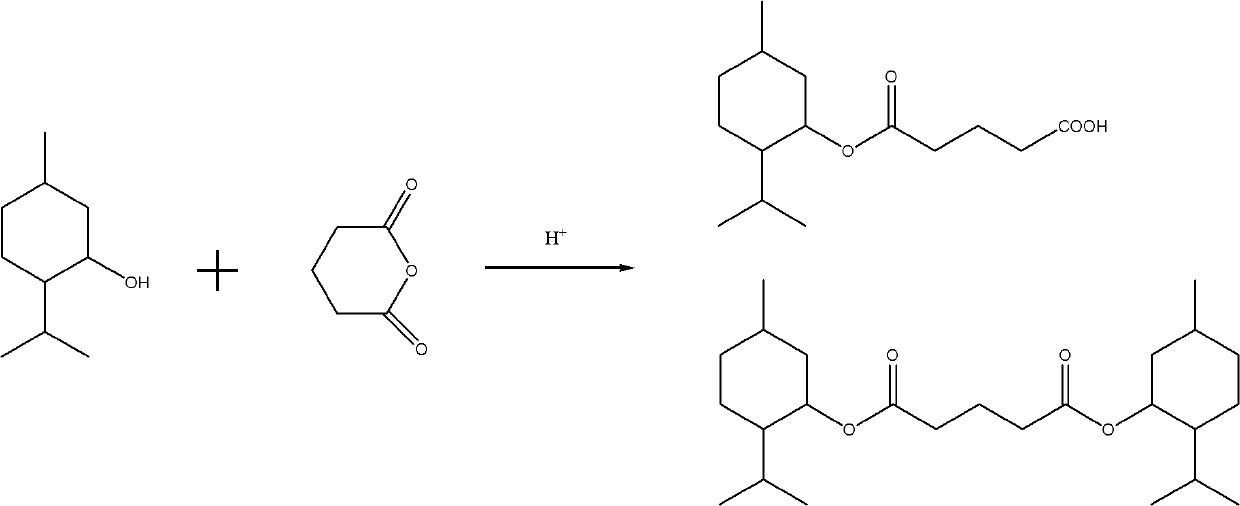
Preparation method for physiological cooling agent menthyl glutarate
A technology of preparing glutaric acid and menthyl ester, which is applied in the field of preparation of physiological cooling agent menthyl glutarate, can solve the problems of few reports on the synthesis of menthyl glutarate, long reaction time, etc., and achieve good aroma and taste , the effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
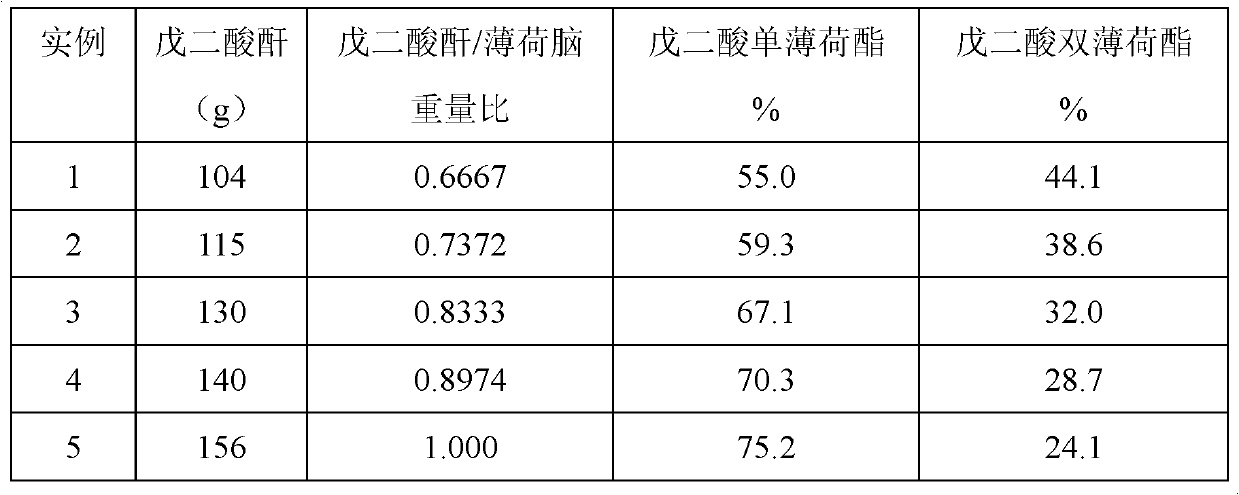
[0012] Add 156 grams of menthol, 104 grams of glutaric anhydride (the weight ratio of glutaric anhydride to menthol is 0.6667:1) and 2 grams of p-toluenesulfonic acid in a 1000ml three-necked flask equipped with stirring, condenser, thermometer and dropping funnel. And 300 grams of heptane, reflux for about 6 hours, the reaction temperature is 50 ~ 110 ℃, chromatographic analysis of menthol content ≤ 1%, cool, wash with brine until neutral, separate the oil layer, recover the solvent, and analyze the remainder by GC , The content of monomenthyl glutarate in the menthyl glutarate mixture is 55.0%, and the content of dimenthyl glutarate is 44.1%.
[0013] Using the method of this embodiment, the content of glutaric acid monomenthyl and dimenthyl glutarate mixtures obtained with different weight ratios of glutaric anhydride to menthol are also different. The analysis results are as follows:
[0014]
Embodiment 2
[0016] The dosage of menthol is the same as in Example 1, except that the acidic catalyst used is phosphoric acid, the dosage is 2 grams, the dosage of glutaric anhydride is 120 grams, the non-polar solvent used in the reaction is 300 grams of toluene, and the reaction is refluxed. The time is 4 hours, and the content of monomenthyl glutarate in the menthyl glutarate mixture is 63.4%, and the content of dimenthyl glutarate is 35.5%.
Embodiment 3
[0018] The feeding amount of menthol is the same as in Example 1, except that the acidic catalyst used is p-toluenesulfonic acid, the feeding amount is 2 grams, the feeding amount of glutaric anhydride is 135 grams, the reaction does not add solvent, and the reaction temperature is controlled to 90°C. The reaction time is 5 hours, and the content of monomenthyl glutarate in the mixture of menthyl glutarate is 69.6%, and the content of dimenthyl glutarate is 29.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com