Method for covalently coupling amino-containing molecules to microspheres
A covalent coupling and amino group-containing technology, which is applied in the field of biochemical and clinical testing, can solve the problems of waste of raw materials, poor linearity of reagents, and affecting the measured value of samples to be tested.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
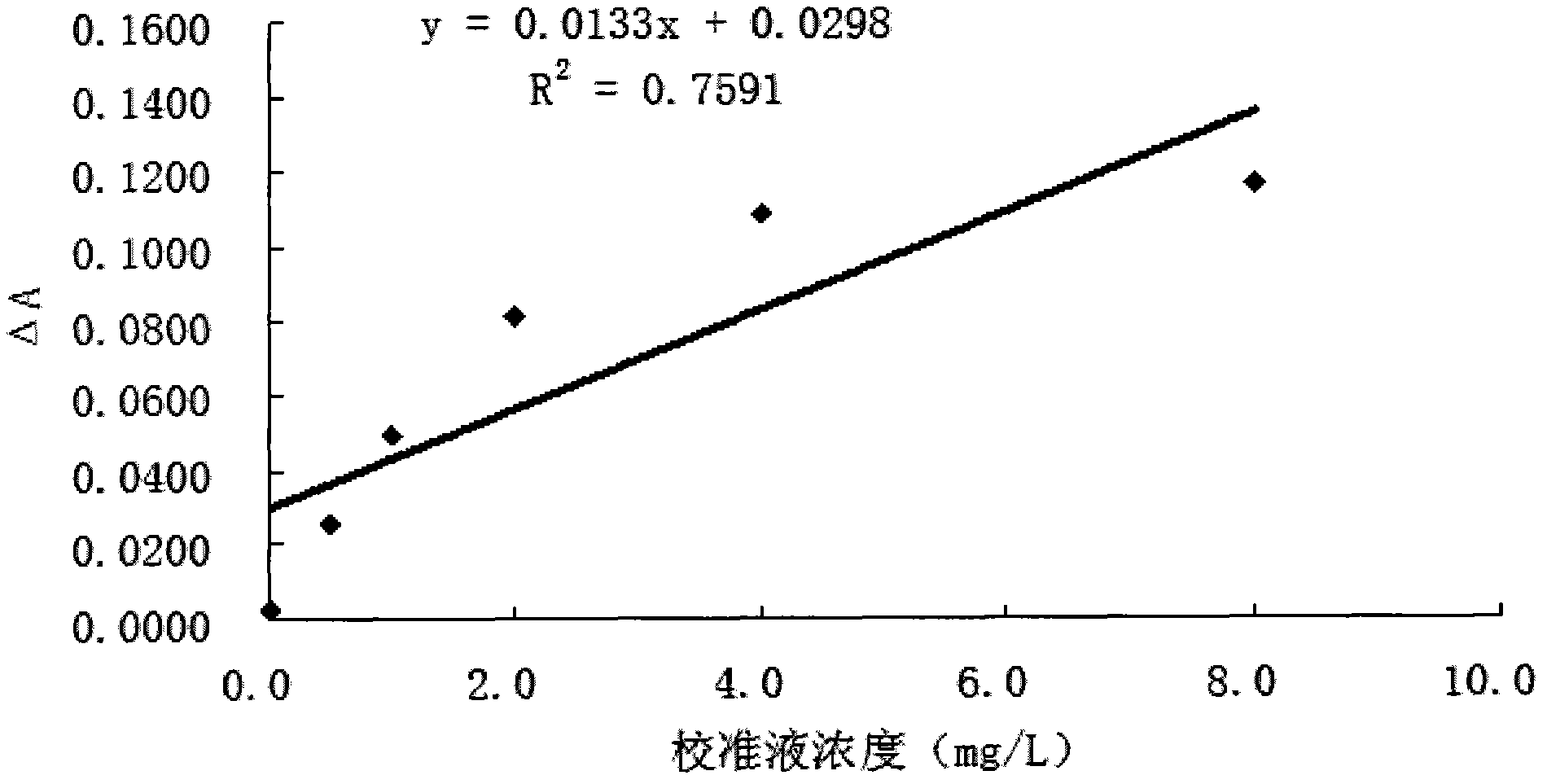
[0057] Example 1: Traditional one-step conjugation of anti-Cys-C antibody
[0058] 1) Take 100 μL of 10% latex particles (150nm), add 2mL of 10mM MES solution (pH6.1) to mix, centrifuge at 15,000rpm for 50min, discard the supernatant, add 2mL of 10mM MES solution (pH6.1) and use a pipette to repeatedly suck The latex particles are dispersed and mixed evenly, and the concentration of the latex particles is 0.5%.
[0059] 2) Take 4.5 μL of rabbit anti-human Cys-C antibody with a concentration of 22 mg / mL, dilute it to 100 μL with PBS buffer solution of pH 7.4, and mix well.
[0060] 3) Mix the microsphere solution obtained in step 1) with 40 μL of the anti-Cys-C antibody solution obtained in step 2), place on a magnetic stirrer with a rotor and stir for 15 minutes to fully mix the two.
[0061] 4) Accurately weigh 0.0050 g of EDC and dissolve in 1 mL of deionized water to obtain 5 mg / mL of EDC, add 25 μL to the mixed solution of latex particles and anti-Cys-C antibody, and keep...
Embodiment 2
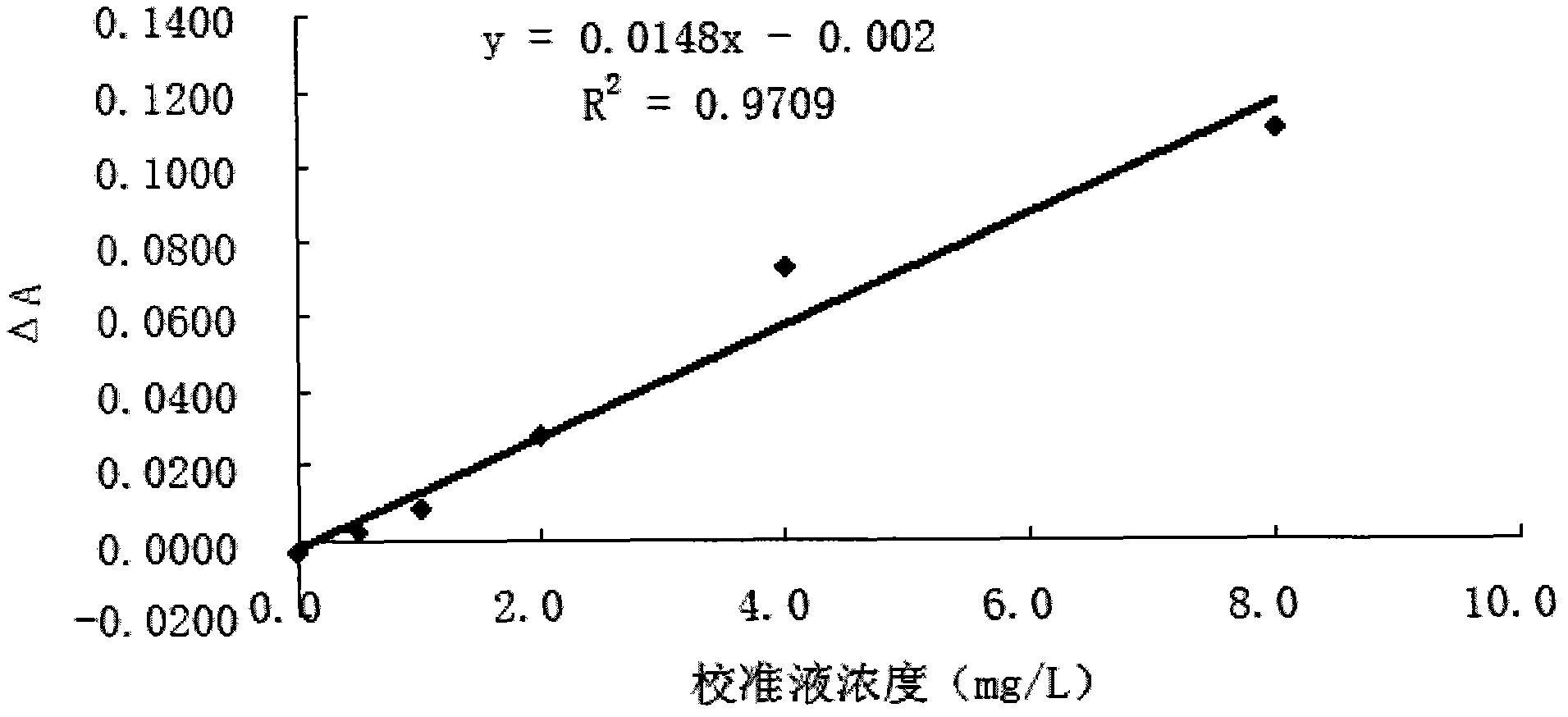
[0063] Embodiment 2: The method of the present invention (using Sulfo-NHS) coupling anti-Cys-C antibody
[0064] 1) Take 100 μL of 10% latex particles (150nm), add 2mL of 10mM MES solution (pH6.1) to mix, centrifuge at 15,000rpm for 50min, discard the supernatant, add 2mL of 10mM MES solution (pH6.1) and use a pipette to repeatedly suck The latex particles are dispersed and mixed evenly, and the concentration of the latex particles is 0.5%.
[0065] 2) Take 4.5 μL of rabbit anti-human Cys-C antibody with a concentration of 22 mg / mL, dilute it to 100 μL with PBS buffer solution of pH 7.4, and mix well.
[0066] 3) Mix the microsphere solution obtained in step 1) with 40 μL of the anti-Cys-C antibody solution obtained in step 2), place on a magnetic stirrer with a rotor and stir for 15 minutes to fully mix the two.
[0067] 4) Add 25 μL EDC (5 mg / mL) together with 25 μL Sulfo-NHS (50 mg / mL) to the mixed solution of latex particles and anti-Cys-C antibody at the same time, and k...
Embodiment 3
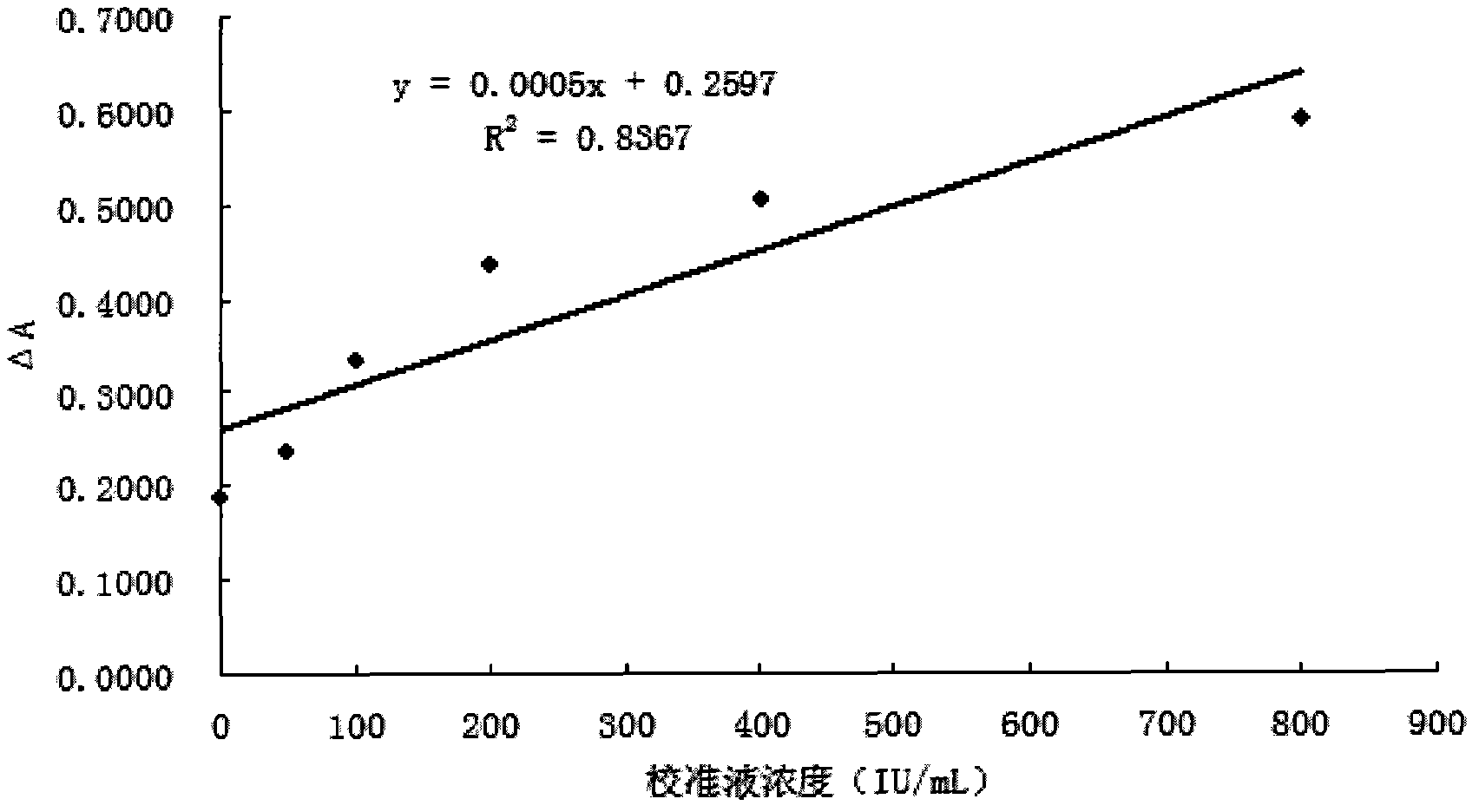
[0069] Embodiment 3: the preparation of Cys-C reagent
[0070] Get the latex particle of embodiment 1 (or 2) harvest and add 2mL0.01M PBS (pH7.4) ultrasonic dispersion and clean; NaN 3 , pH8.4) ultrasonic dispersion to obtain latex particles with a concentration of 0.2%; after magnetic stirring for 30 min, store at 2-8°C until use.
[0071] The formula is as follows:
[0072] 0.1M glycine buffer pH8.4;
[0073] 0.5% BSA;
[0074] 0.2% microspheres coupled with anti-Cys-C antibody;
[0075] 0.05%NaN 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com