Method for preparing phenylethylphenylethane capacitor insulating oil by solid acid catalysis
A technology catalyzed by phenylethylphenylethane and solid acid, which is applied in base materials, organic chemistry, petroleum industry, etc., can solve the problems of complex catalyst regeneration process, inability to recycle, catalyst deactivation, etc., and achieve catalyst activity maintenance The effect of constant, high selectivity and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
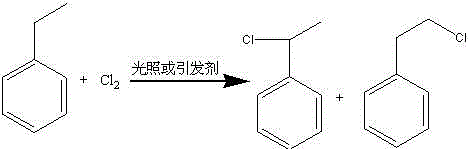
[0025] Add 4 mol of ethylbenzene to reactor A, and under the action of stirring, 120°C and ultraviolet light irradiation, feed 1 mol of chlorine gas into reactor A at a constant speed for 3 hours. The hydrogen chloride gas generated in the process is absorbed by water to obtain the by-product hydrochloric acid to be discharged, and cooled to room temperature to obtain a mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene;
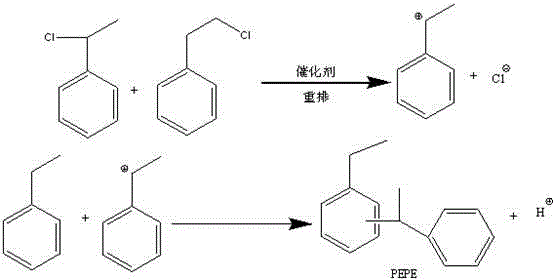
[0026] Add the mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene in the reactor A to a mixture of 5mol ethylbenzene and 10g JHSK-8 gel-type strong acid cation at 60°C at one time Reaction was carried out in the reactor B of exchange resin, 6g mordenite mixture; After mixing, continue to react at 80 ℃ for 4 hours, after gas chromatography detection α-chloroethylbenzene and β-chloroethylbenzene are completely consumed, Stop the reaction, absorb the hydrogen chloride gas generated in the reaction process with water, get ...
Embodiment 1-2
[0029] Add 5 mol of ethylbenzene into reactor A, under stirring, 110°C and 5 g of azobisisobutyronitrile, 1 mol of chlorine gas is passed into reactor A at a constant speed for 3 hours, after the chlorine gas is passed through, continue the reaction for 2 hour, the hydrogen chloride gas generated in the reaction process is absorbed by water to obtain the discharge of by-product hydrochloric acid, and is down to room temperature, and the mixed material of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene obtained;
[0030]Add the mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene in the reactor A to the reactor B containing the mixture of 5mol ethylbenzene and 20g zeolite β at 60°C at one time Carry out the reaction; after the mixing is completed, continue the reaction at 70°C for 8 hours, and stop the reaction after the gas chromatography detects that α-chloroethylbenzene and β-chloroethylbenzene are completely consumed, and absorb the hydrogen chl...
Embodiment 1-3
[0033] Add 6 mol of ethylbenzene into reactor A, and under stirring, 130°C and ultraviolet light irradiation, 1 mol of chlorine gas is passed into reactor A at a constant speed for 4 hours. After the chlorine gas is passed through, continue to react for 1 hour. The hydrogen chloride gas generated in the process is absorbed by water to obtain the by-product hydrochloric acid and discharged, and kept warm to obtain a mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene;
[0034] Add the mixture of α-chloroethylbenzene, β-chloroethylbenzene and ethylbenzene in Reactor A to a mixture containing 10mol ethylbenzene, 5g L-type zeolite, 10g ZSM-12 at 100°C at one time The mixture was reacted in Reactor B; after the mixing was completed, the reaction was continued at 100°C for 8 hours, and after the complete consumption of α-chloroethylbenzene and β-chloroethylbenzene was detected by gas chromatography, the reaction was stopped. During the reaction The generated hydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com