Preparation method of hindered amine light stabilizer
A hindered amine light stabilizer and solvent technology, which is applied in the field of preparation of hindered amine light stabilizers, can solve the problems of complex operation, high cost, and high price, and achieve the effects of simple operation, lower price, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
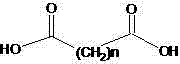
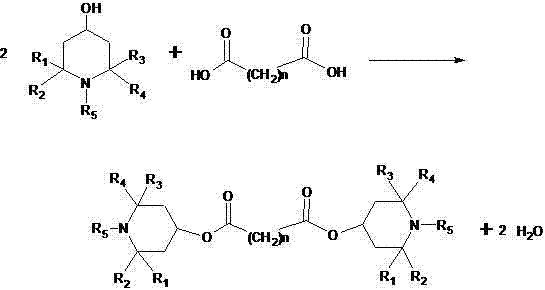
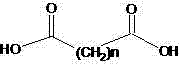
[0026] Example 1: The preparation method of a hindered amine light stabilizer in this example, add 200ml of solvent naphtha to a 500ml flask, then add the raw materials sebacic acid (20.2g 0.1mol) and 2,2,6,6-tetra Methylpiperidinol (34.6g 0.22mol) and catalyst, warmed to reflux. After 10-15 hours, the reaction ends. The temperature is lowered to precipitate, and the separated solvent is reused. Washed with water and further refined to obtain pure bis(2,2,6,6-tetramethyl-4-piperidinyl) sebacate with a melting point of 81-83°C, with a yield of 80.5%
Embodiment 2
[0027] Example 2: The preparation method of a hindered amine light stabilizer of this example, add 200ml of the recovered solvent oil of Example 1 in a 500ml flask, and then add the raw materials sebacic acid (20.2g 0.1mol) and 2,2, 6,6-tetramethylpiperidinol (0.22mol) and catalyst, heated to reflux for reaction. After 10-15 hours, the reaction ends. The temperature is lowered to precipitate, and the separated solvent is reused. Wash with water, put the crude product into solvent oil and add activated carbon for decolorization and recrystallization to obtain bis(2,2,6,6-tetramethyl-4-piperidinyl) sebacate with a main content>98%, and the yield is 94.0 %.
Embodiment 3
[0028] Example 3: The preparation method of a hindered amine light stabilizer in this example, add 200ml of solvent naphtha to a 500ml flask, then add the raw material sebacic acid (20.2g 0.1mol) and 1, 2, 2, 6, 6 -Pentamethylpiperidinol (0.22mol) and catalyst, heated to reflux reaction. After 10-15 hours of reaction, the color is washed with water, and the solvent is distilled off for reuse. Bis(1,2,2,6,6-pentamethyl-4-piperidinyl) sebacate was obtained with a yield of 90.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com