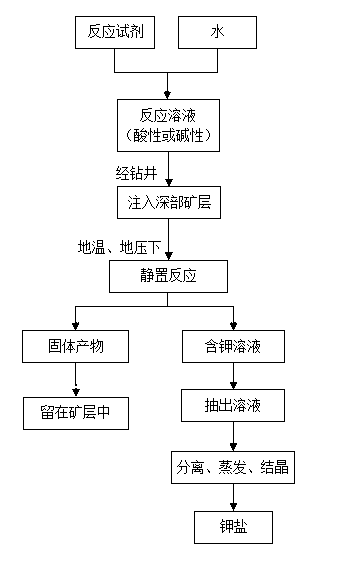
Method for preparing water-soluble sylvite from deep polyhalite in in-situ reaction mode
A polyhalite ore and in-situ reaction technology, which is applied in earth square drilling, wellbore/well components, mining fluids, etc., can solve the problem of large-scale extraction of potassium in deep polyhalite mines, difficulty in crushing, and daily outflow Small volume and other problems, to avoid processing problems, simple process, large injection volume effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A simulated test for in-situ reaction of polyhalite ore with a buried depth of 2800m (the ground temperature is about 90°C and the ground pressure is about 28MPa) to prepare water-soluble potassium salts, which includes the following steps:
[0032] The simulated test was carried out with Dalong polyhalite ore in Guang'an, Sichuan. The ore K 2 O content is 12.64wt%;
[0033] (1) Use 0.5wt% HCl aqueous solution as the reaction solution.
[0034] (2) Put 200g of dense massive polyhalite ore into a high-temperature and high-pressure reaction kettle, and add 500ml of reaction solution to ensure that the reaction solution is completely soaked in polyhalite ore.
[0035] (3) Use a high-temperature and high-pressure reactor to simulate the deep underground ground temperature and ground pressure conditions, set the reaction temperature to 90°C, and the reaction pressure to 28MPa.
[0036] (4) Heat and pressurize to the set temperature and pressure conditions, let it stand for...
Embodiment 2
[0042] A simulated test for in-situ reaction of polyhalite ore with a buried depth of 2000m (the ground temperature is about 70°C and the ground pressure is about 20MPa) to prepare water-soluble potassium salt, the steps are as follows:
[0043] (1) The ore of the Wusheng Wanshan polyhalite mine in Guang'an was tested, and the ore K 2 The O content is 11.38 wt%.
[0044] (2) The NaOH aqueous solution with a concentration of 5 wt% was used as the reaction solution.
[0045] (3) Put 200g of dense massive polyhalite ore into a high-temperature and high-pressure reaction kettle, and add 500ml of reaction solution to ensure that the reaction solution completely soaks the polyhalite ore.
[0046] (4) Use a high-temperature and high-pressure reactor to simulate the deep underground ground temperature and ground pressure conditions, set the reaction temperature to 70°C, and the reaction pressure to 20MPa.
[0047] (5) Heat and pressurize to the set temperature and pressure condition...
Embodiment 3
[0053] A simulated test for in-situ reaction of polyhalite ore with a buried depth of 1000m (the ground temperature is about 45°C and the ground pressure is about 10MPa) to prepare water-soluble potassium salts. The steps are as follows:
[0054] (1) The ore of Nongle polyhalite ore in Quxian County, Sichuan Province was tested, and the ore K 2 The O content is 12.83 wt%.
[0055] (2) With a concentration of 5 wt% H 2 SO 4 The aqueous solution is the reaction solution.
[0056] (3) Put 200g of dense massive polyhalite ore into a high-temperature and high-pressure reaction kettle, and add 500ml of reaction solution to ensure that the reaction solution completely soaks the polyhalite ore.
[0057] (4) Use a high-temperature and high-pressure reactor to simulate the deep underground ground temperature and ground pressure conditions, set the reaction temperature to 45°C, and the reaction pressure to 10MPa.
[0058] (5) Heating and pressurizing to the set temperature and pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com