Anti CCR4 antibodies and uses thereof
A technology of antibodies and antagonists, applied in the direction of antibodies, anti-inflammatory agents, anti-bacterial drugs, etc., can solve problems such as lack of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0476] Example 1: New Antibodies
[0477] Nine human antibodies capable of specifically binding CCR4 have been identified. The single chain form of the antibody was cloned into the pHOG21 plasmid, which contains c-myc and 6 his-tagged epitopes. TG1 bacteria were transfected and scFv expression was induced with IPTG. Binding of purified scFv was confirmed by EasyCyte (see Example 2).
[0478] The nucleotide sequences of the heavy and light chains of the resulting cloned antibodies were sequenced. The antibodies are designated as 208, 306, 308, 406, 501, 503, 601, 603 and 803. The CDR regions of the light and heavy chains of 208, 306, 308, 406, 501, 503, 601, 603 and 803 The iso-sequences are shown in Tables 1-9, respectively.
[0479] Antibodies 208, 306, 308, 406, 501, 503, 601, 603 and 803 were also prepared in IgG form. IgG was prepared using standard protocols. Briefly, the genes encoding the corresponding variable domains were cloned into the mammalian expression v...
Embodiment 2
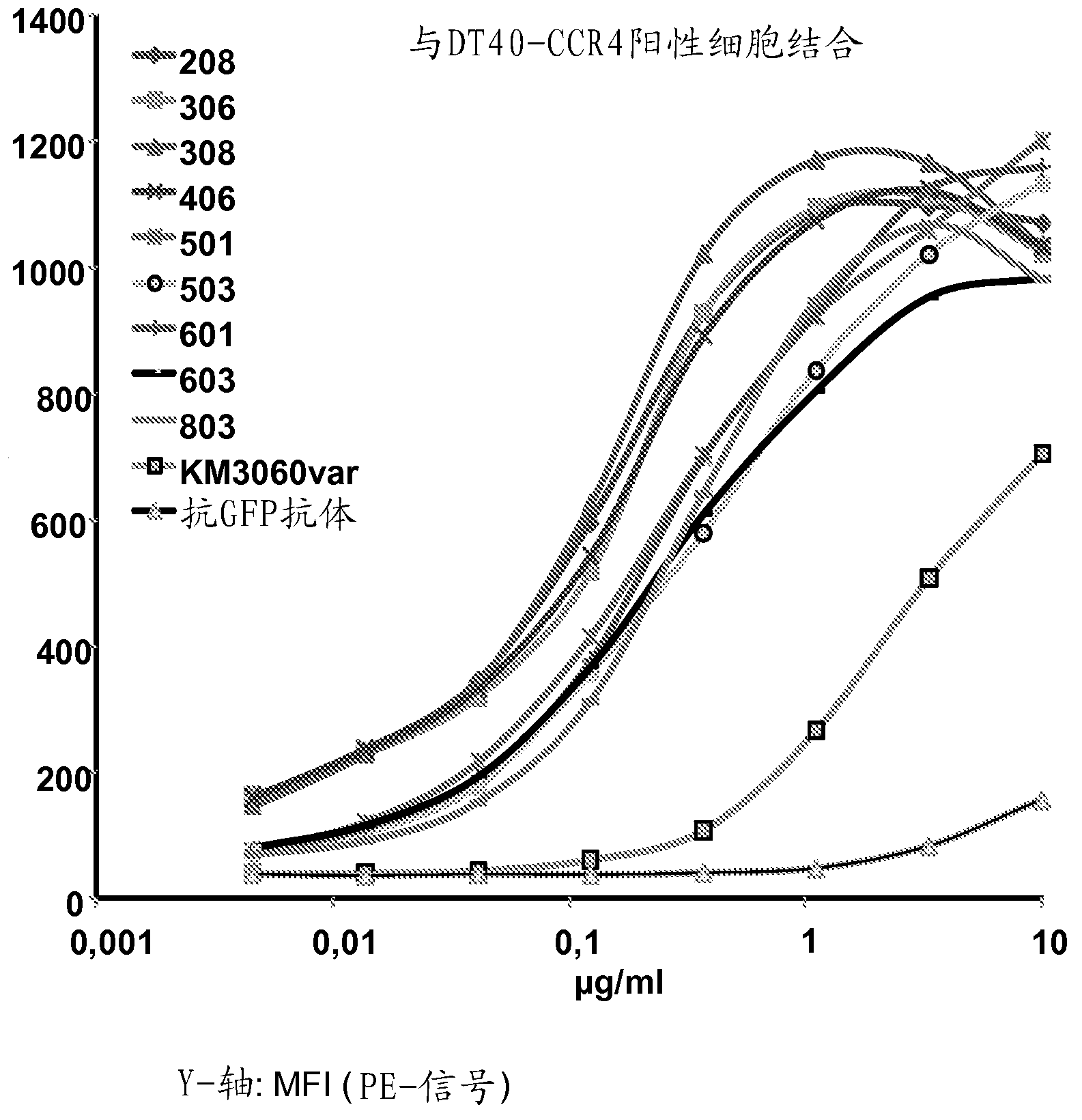
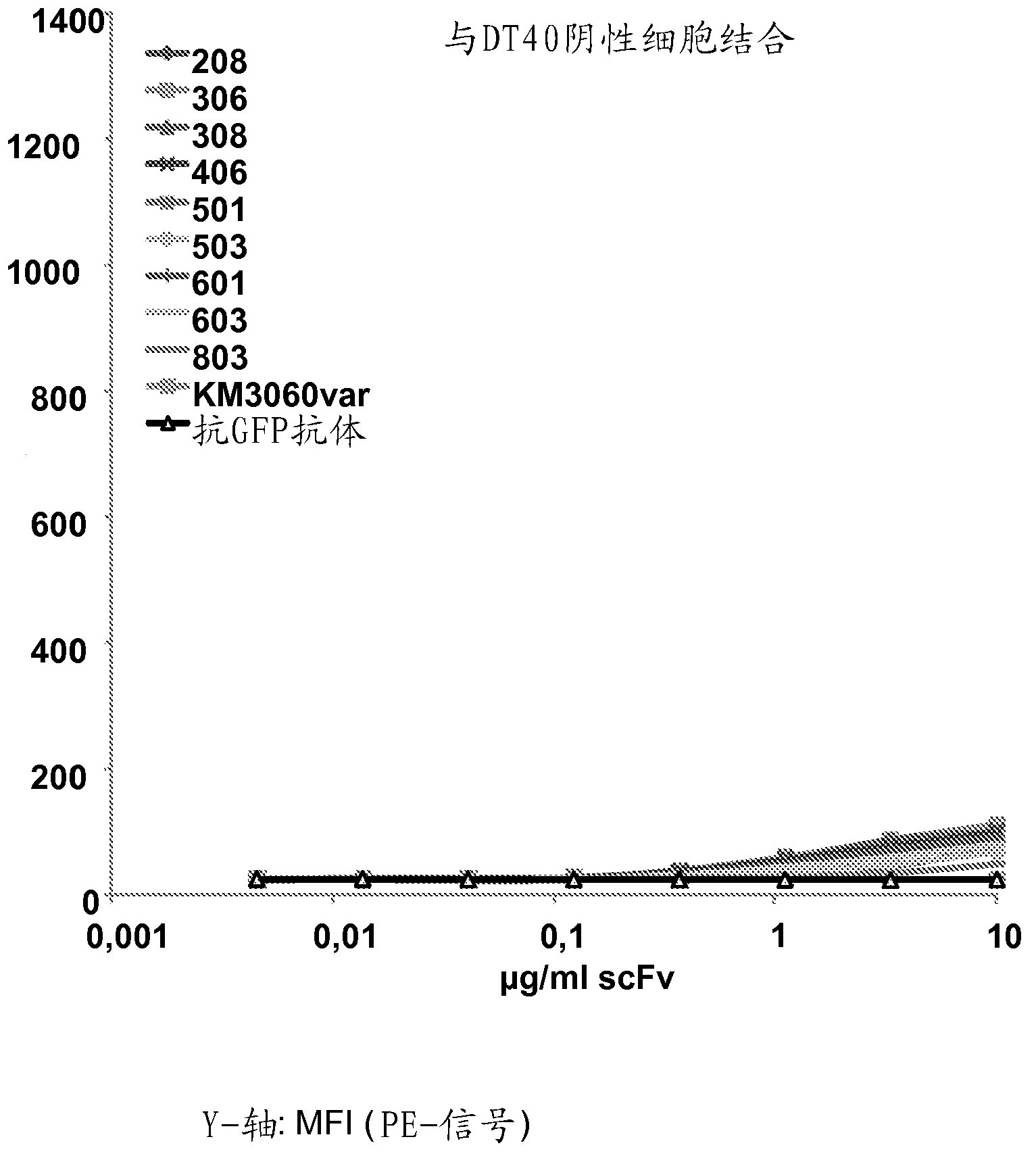
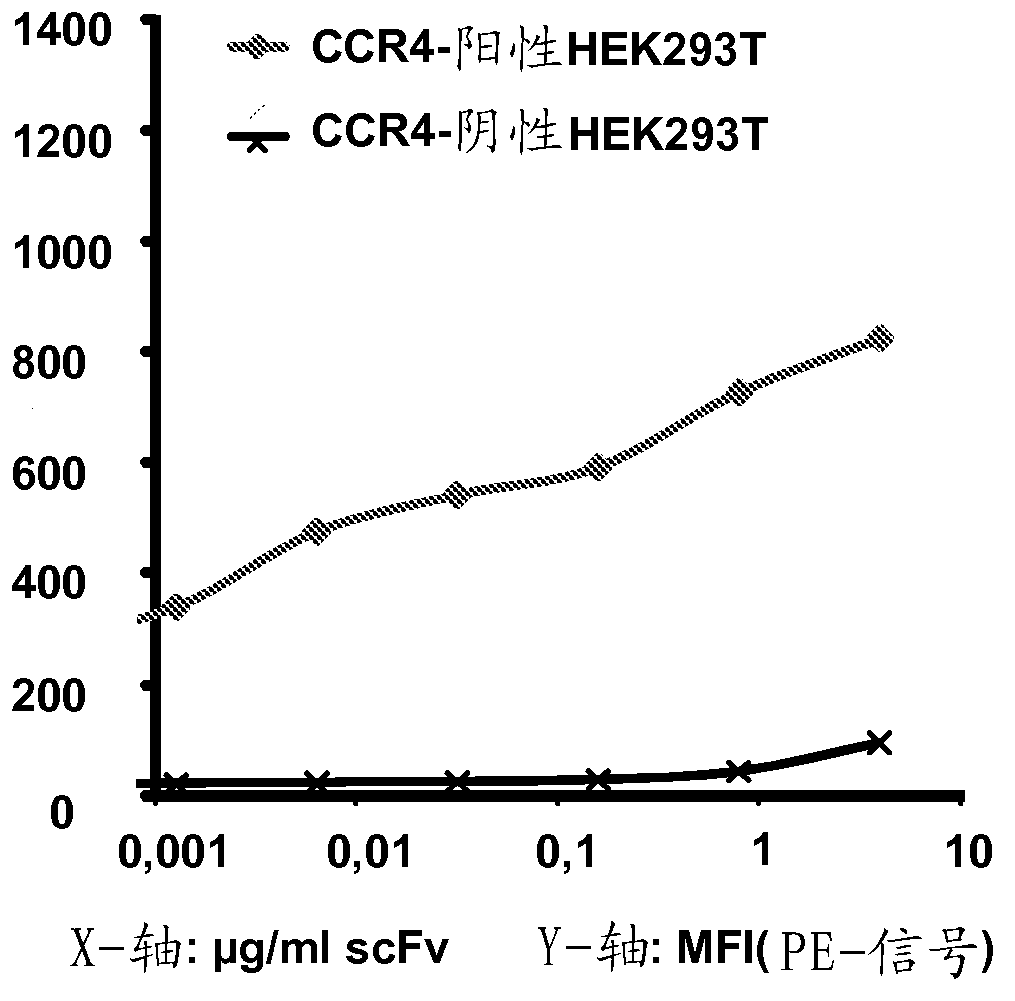
[0481] Example 2: Binding of Anti-CCR4 Antibodies to Target Expressing Cells
[0482] To demonstrate the CCR4 specificity of the antibodies disclosed in Example 1, scFv208, 306, 308, 406, 501, 503, 601, 603 and 803 were used in flow cytometry for internally transfected with CCR4 and not transfected with CCR4. HEK293T cells, DT40 cells and native CCR4 + CCRF-CEM cell line staining. An in-house cloned and expressing KM3060var scFv was used as a positive control. Anti-GFP scFv antibody (raised against green fluorescent protein) was used as a negative control.
[0483] CCRF-CEM (acute lymphocytic leukemia, ATCC No. CCL-119), HEK293T / 17 (human kidney, ATCC No. CRL-11268), and DT40 (chicken lymphoma, ATCC No. CRL-2111) cell lines were cultured from American models Acquired from the Art Collection (ATCC, Rockville, MD).
[0484] For transient transfection with human CCR4, Hek293T / 17 cells were seeded in T75 (NUNC) flasks at 2 × 10 6 cells. 48 hours after seeding, the cells we...
Embodiment 3
[0503] Example 3: Anti-CCR4 Antibodies Interfering with Ligand Binding
[0504] To determine whether the anti-CCR4 antibodies from Example 1 interfere with CCR4 ligand and receptor binding, competition experiments were performed. To this end, CCR4-positive CCRF-CEM target cells were incubated at a fixed concentration of MDC-SNAP in the presence of increasing concentrations of scFv. The MDC is genetically fused to the N-terminus of the SNAP tag such that the SNAP tag is fused to the C-terminus of the MDC. The SNAP tag is derived from the 20 kDa DNA repair protein O6 alkylguanine-DNA alkyltransferase. The gene encoding the MDC-SNAP fusion protein was custom made at Geneart (Regensburg, Germany) and HEK293 / T cells were transiently transfected with the gene. After 5-6 days, the fusion protein was purified by a Ni-NTA affinity column, followed by isolation of a single-section MDC-SNAP fragment by steric exclusion. MDC-SNAP was labeled with Alexa647 according to the procedure i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com