Preparation method of azodiisobutyronitrile
A technology for dichlorination of azobisisobutyronitrile and acetone cyanamide, which is applied in the field of preparation of azobisisobutyronitrile, can solve the problems that the reaction end point is not easy to control, the by-products are not easily separated, and the production cost is high, and the production process is achieved. Green environmental protection, easy control of the reaction end point, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
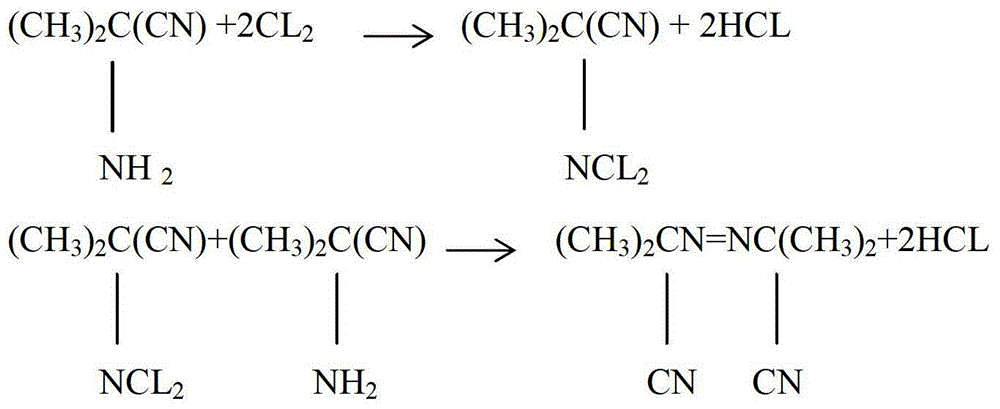
[0025] Preparation of acetone cyanamide dichloride: Add 260 grams of pure water and 85 grams of 99% acetone cyanamide in a 1000ml three-neck flask, pass chlorine gas under stirring, observe the situation of chlorine gas bubbles being absorbed, judge the end point of the reaction, pass 0.5 hours After completion, the reaction temperature is controlled at 15°C during the process of chlorine flow. The final amount of chlorine flow is obtained by weighing the weight of the material in the three-necked flask, and the controlled amount of chlorine flow is 149±7 grams. Add 260 grams of pure water and 85 grams of acetone cyanamide), add 30% liquid caustic soda dropwise, control the dropping temperature at 15°C during the dropping process, and finish dropping in 0.5 hours. Use a pH meter to track and detect to ensure that the pH value is 7.
[0026] The preparation of azobisisobutyronitrile: in the above-mentioned three-necked flask, dropwise add 80 grams of acetone cyanamide and 30% li...
Embodiment 2
[0029] Preparation of acetone cyanamide dichloride: Add 255 grams of pure water and 85 grams of 99% acetone cyanamide in a 1000ml three-neck flask, pass chlorine gas under stirring, observe the situation of chlorine gas bubbles being absorbed, judge the end point of the reaction, pass 1 hour After completion, the reaction temperature is controlled at 30°C during the chlorine flow process. The final chlorine flow rate is obtained by weighing the material weight in the three-necked flask. The controlled chlorine flow rate is 160±3 grams. Add 255 grams of water and 85 grams of acetone cyanamide), add dropwise 30% liquid caustic soda, control the dropwise addition temperature at 25°C during the dropwise addition, and finish dropping in 1 hour. Use a pH meter to track and detect to ensure that the pH value is 8.
[0030] Preparation of azobisisobutyronitrile: Add 80 grams of acetone cyanamide and 30% liquid caustic dropwise to the above-mentioned three-necked bottle at the same time...
Embodiment 3
[0033] Preparation of acetone cyanamide dichloride: add 340 grams of pure water and 85 grams of 99% acetone cyanamide to a 1000ml three-neck flask, pass chlorine gas under stirring, observe the situation of chlorine gas bubbles being absorbed, judge the reaction end point, pass through in 0.8 hours, During the process of chlorine flow, the reaction temperature is controlled at 20°C. The final amount of chlorine flow is obtained by weighing the weight of the material in the three-necked flask. The controlled amount of chlorine flow is 167±3 grams. gram of water and 85 grams of acetone cyanamide) was added dropwise with 30% liquid caustic soda, the dropping temperature was controlled at 20°C during the dropping process, the dropping was completed in 0.9 hours, and the pH meter was used to track and detect to ensure that the pH value was 7.5.
[0034] The preparation of azobisisobutyronitrile: in the above-mentioned three-necked flask, dropwise add 80 grams of acetone cyanamide an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com