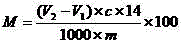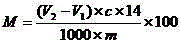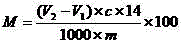Method for titrating primary amine content of natural protein fibers
A protein fiber, primary amine technology, applied in material analysis by observing the influence of chemical indicators, and analysis by chemical reaction of materials, etc., can solve the problem that natural protein fibers cannot be applied, and the molecular structure of natural protein fibers is destroyed. , inaccurate results, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A Esterification of wool
[0056] Submerge 1 g of the cleaned wool in acetone with a bath ratio of 1:50, add 0.02 g of a silane coupling agent whose organophilic group is an alkane long molecular chain, and carry out esterification reaction with the extracted wool at a reaction temperature of 70 °C. After 3 hours of reaction, a Soxhlet extractor was used to remove unreacted silane coupling agent. The extraction agent was acetone, and the extraction temperature was 80 oC. After 3 hours of extraction, it was placed in a vacuum oven for drying. Mpa, after drying for 5h, wool is obtained after esterification;
[0057] B Reaction of wool with MDI after esterification
[0058] Put the esterified wool obtained in step A into toluene, the bath ratio is 1:20, add 0.5gMDI to react with the esterified wool, the reaction temperature is 35 oC, and take the reacted wool out of toluene after 20 minutes of reaction , using xylene to clean the reacted wool, the bath ratio is 1:10, the...
Embodiment 2
[0072] A. Esterification of cashmere
[0073] Immerse 1 g of cashmere after cleaning in acetone, the bath ratio is 1:60, add 0.017 g of silane coupling agent whose organophilic group is methyl and carry out esterification reaction with the cashmere after cleaning, the reaction temperature is 75 oC, after 4 hours of reaction Use a Soxhlet extractor to remove unreacted silane coupling agent. The extraction agent is acetone. The extraction temperature is 80 oC. After extraction for 4 hours, it is placed in a vacuum oven for drying. The drying temperature is 135 oC and the vacuum degree is -0.1Mpa. After 6h, obtain cashmere after esterification;
[0074] B. Reaction of cashmere and TDI after esterification
[0075] Put the cashmere after esterification obtained through step A into xylene, the bath ratio is 1:25, add 0.33g TDI to react with the cashmere after esterification, the reaction temperature is 40 ℃, react the cashmere after reaction for 30min from two Take out from tolue...
Embodiment 3
[0089] A Esterification reaction of silk
[0090] Immerse 1g of silk after cleaning in acetone, the bath ratio is 1:70, add 0.014g of silane coupling agent whose organophilic group is vinyl to carry out esterification reaction with silk after cleaning, the reaction temperature is 8-0 oC, the reaction After 5 hours, use a Soxhlet extractor to remove the unreacted silane coupling agent. The extraction agent is acetone, and the extraction temperature is 80 oC. After 5 hours of extraction, it is placed in a vacuum oven for drying. , after drying for 7 hours, the esterified silk was obtained;
[0091] B Reaction of silk and HDI after esterification
[0092] Put the esterified silk obtained in step A into acetone, the bath ratio is 1:30, add 0.2g HDI to react with the esterified silk, the reaction temperature is 45 oC, after 40min, take the reacted silk out of the acetone , using xylene to clean the silk after the reaction, the bath ratio is 1:30, the cleaning temperature is 45 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com