Application of doxycycline in preparing medicines for enhancing PAC1-R (Pituitary Adenylate Cyclase Activating Polypeptide Type 1 Receptor) dimer dependent constitutive activity
A doxycycline, inherently active technology, applied in drug combinations, active ingredients of tetracycline, diseases, etc., can solve the problems that the target of action has not been elucidated, and the mechanism of action of doxycycline is unclear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Doxycycline promotes the proliferation of PAC1-CHO cells expressing PAC1-R dimers
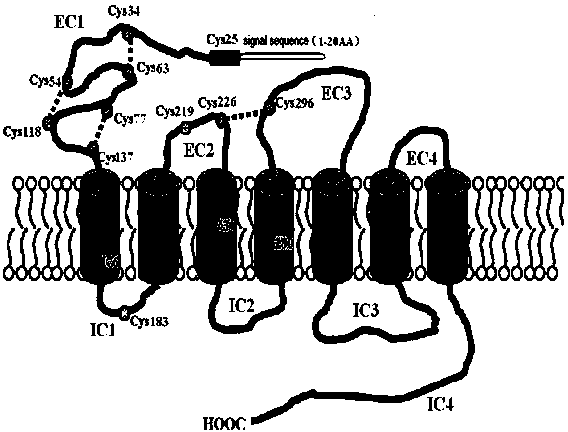
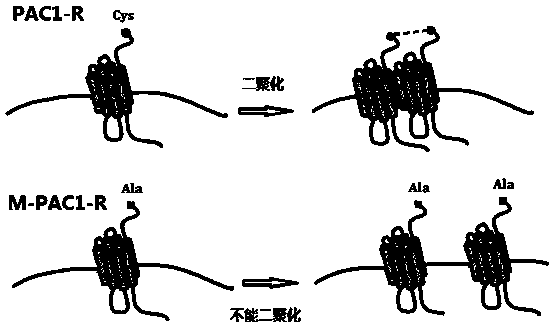
[0027] Published articles (PLoS One. 2012;7(12):e15811) have clarified that PAC1-R can form dimers, and the formation of dimers depends on the first Cys at the N-terminal (position 25), if By mutating Cys to Ala, the mutant M-PAC1-R cannot dimerize. The structural model of PAC1-R is shown in the figure figure 1 ; PAC1-R and M-PAC1-R dimer model diagram figure 2 .
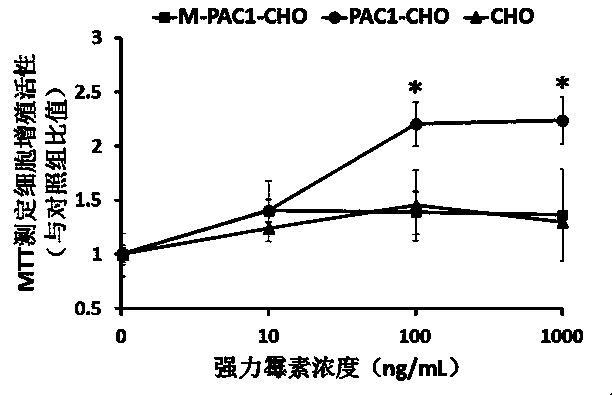
[0028] The established CHO cell line PAC1-CHO stably expressing PAC1-R and the CHO cell M-PAC1-CHO stably expressing Cys / Ala mutant M-PAC1-R were used in the experiment. The effect of doxycycline on the proliferation activity of the two cell lines was determined by MTT assay. Results (such as image 3 ) found that after incubating cells with 100-1000ng / mL doxycycline for 48 hours, the cell viability was measured by MTT method, and the ratio of cell viability compared with the untreated control group was c...
Embodiment 2
[0029] Example 2 Comparison of doxycycline and PAC1-R agonist maxadilan
[0030] In order to further determine whether doxycycline is an agonist of PAC1-R, the specific agonist maxadilan (1nM) of PAC1-R was used to compare the activity of doxycycline (100ng / mL). The results are as follows Figure 4 As shown, after incubating the cells for 48 hours, the cell viability was measured by MTT method, and it was found that both PAC1-CHO and M-PAC1-CHO were positive for the PAC1-R specific agonist maxadilan (P<0.01, PAC1-CHO treated with maxadilan and M-PAC1 -PAC1-CHO vs PAC1-CHO and M-PAC1-CHO of the blank control), and maxadilan has a more significant effect on the proliferation of M-PAC1-CHO (may be related to dimer steric hindrance that hinders ligand binding); and strong Mycin only had proliferative effects on PAC1-CHO, but basically had no effect on M-PAC1-CHO (P<0.01, PAC1-CHO vs. M-PAC1-CHO and CHO). This result indicates that doxycycline is not an agonist of PAC1-R, but ac...
Embodiment 3
[0031] Example 3 Doxycycline promotes anti-apoptosis of PAC1-CHO cells
[0032] The auxotrophic-induced apoptosis model was prepared by serum-free culture, and the effect of doxycycline on the anti-apoptotic activity of cells expressing PAC1-R dimer was detected. The result is as Figure 5 As shown, after 24 hours of serum-free culture, the activities of the three types of cells all decreased significantly, the residual activity of M-PAC1-CHO cells was 41.6±11.6%, the residual activity of PAC1-CHO was 51.4±8.8%, and the residual activity of CHO was 33.3±10.4%; When the serum was removed and doxycycline (100ng / mL) was added to culture, the residual activity of PAC1-CHO cells was significantly increased to 74.5±9.4% (P<0.01, serum-doxycycline+ group vs. serum-doxycycline-group) . However, the same concentration of doxycycline did not promote the anti-apoptotic function on M-PAC1-CHO and CHO that could not produce PAC1-R dimer. It can be seen that doxycycline only has an ef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com