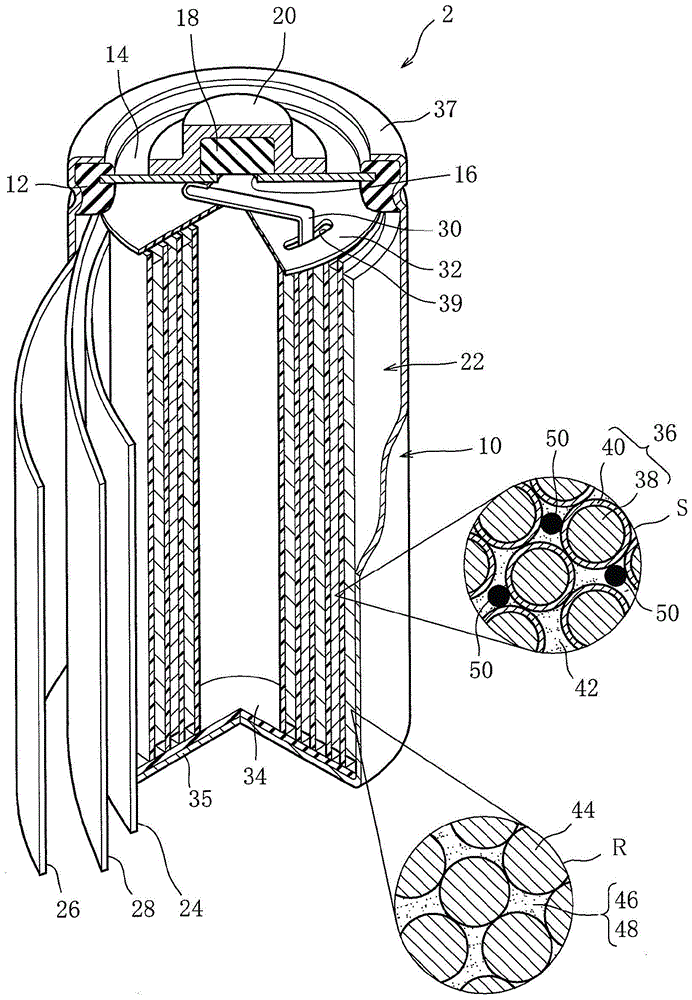
Alkaline battery
一种蓄电池、碱性的技术,应用在碱性蓄电池领域,能够解决难以同时提高自放电特性正极活性物质等问题,达到提高自放电特性、提高利用率、利用率提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 1. Manufacturing of batteries
[0074] (1) Production of positive electrode
[0075] Nickel sulfate, zinc sulfate and cobalt sulfate are weighed so that zinc becomes 4% by weight and cobalt becomes 1% by weight for nickel, and they are added to a 1N (normal concentration) sodium hydroxide aqueous solution containing ammonium ions, Adjust the mixed aqueous solution. While stirring the resulting mixed aqueous solution, gradually add 10N (normal concentration) sodium hydroxide aqueous solution to react in this mixed aqueous solution. In the reaction here, the pH is stabilized at 13 to 14, and nickel hydroxide Base particle 38 in which zinc and cobalt are solid-dissolved as a main body.
[0076] The obtained base particles 38 were washed three times with 10 times the amount of pure water, then dehydrated and dried. In addition, the obtained base particles 38 had a spherical shape with an average particle diameter of 10 μm.
[0077] Next, the obtained base particles 38 w...
Embodiment 2、3
[0092] (embodiment 2,3, comparative example 1,2)
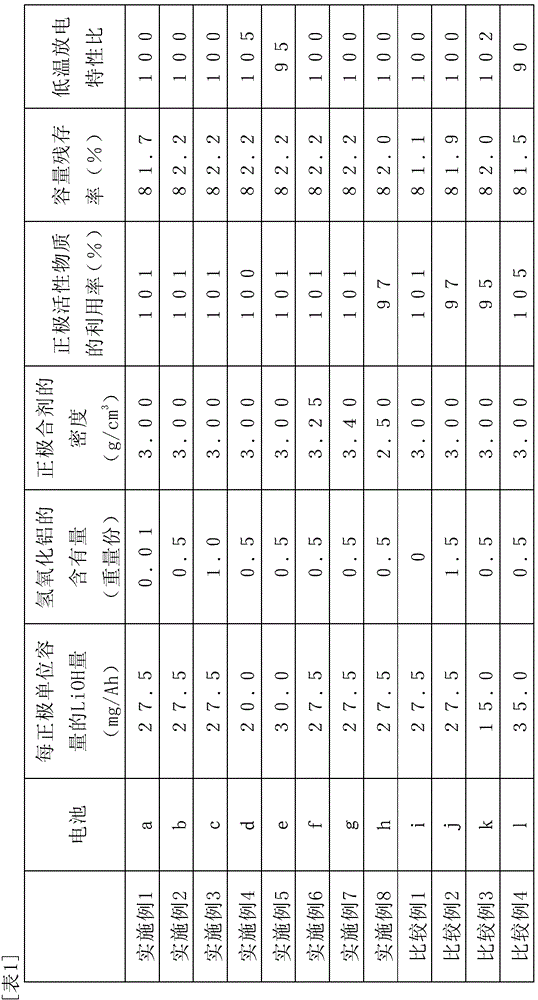
[0093] Appropriately change the amount of aluminum hydroxide added in the positive electrode mixture paste. Table 1 represents the weight part of the content of aluminum hydroxide in the positive electrode for 100 parts by weight of the positive electrode active material powder, except that shown in table 1. Except for this value, nickel-metal hydride storage batteries (batteries b, c, i, j) were produced in the same manner as battery a in Example 1.
Embodiment 4、5
[0094] (embodiment 4,5, comparative example 3,4)
[0095] The concentration of the lithium hydroxide aqueous solution sprayed on the intermediate product particles was appropriately changed, and the amount of LiOH contained in the battery was shown in Table 1 (the value obtained as the amount of LiOH per positive electrode unit capacity), except for the amount of LiOH shown in Table 1. The values other than the values were the same as those of battery b in Example 2, and nickel-hydrogen storage batteries (batteries d, e, k, and 1) were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com