Diagnostic and therapeutic methods
A technology of amyloidosis and active agents, applied in the field of diagnostic methods and kits, pharmaceutical compositions for treatment or prevention, and able to solve problems such as the unclear role of HS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0069] It is obvious to a person skilled in the art that, as technology advances, the inventive concept can be implemented in various ways. The invention and its embodiments are not limited to the examples described below but vary within the scope of the claims.
[0070] Materials and methods
[0071] Aggregation of TTR
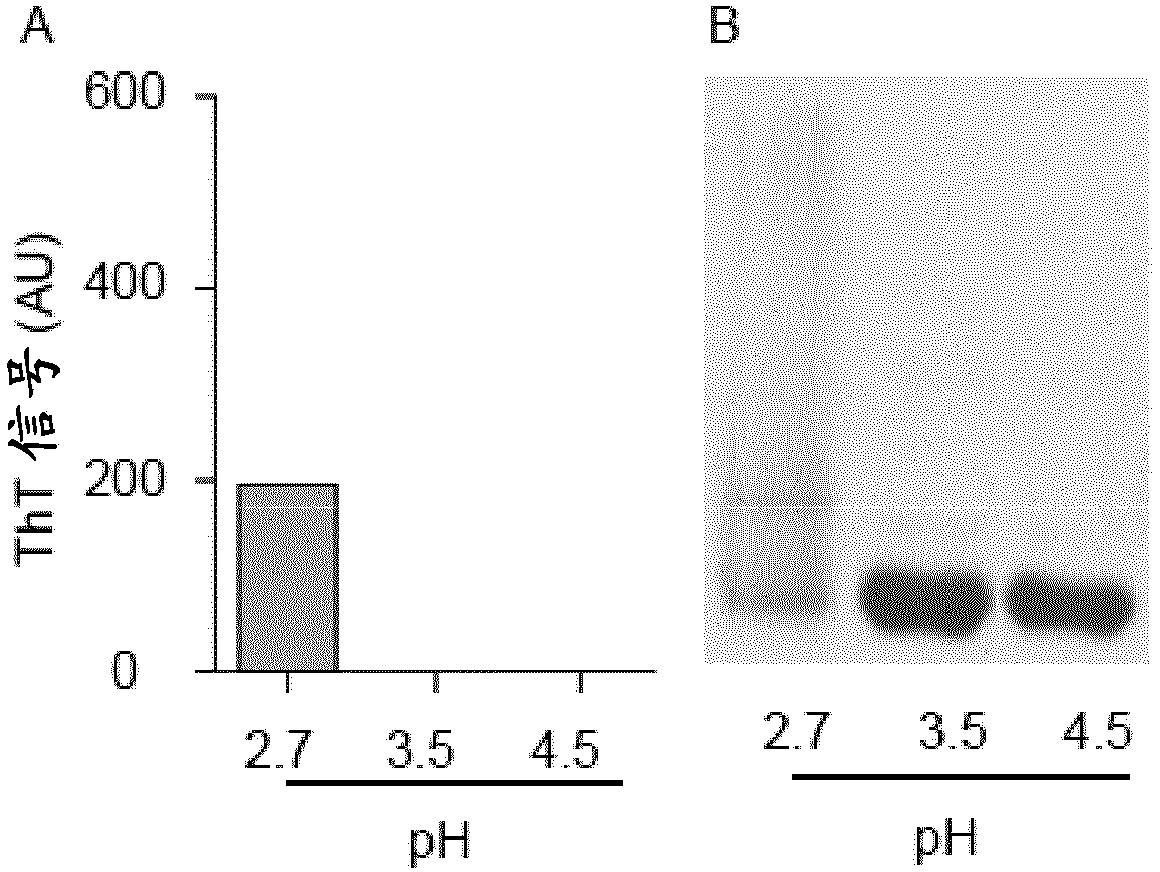
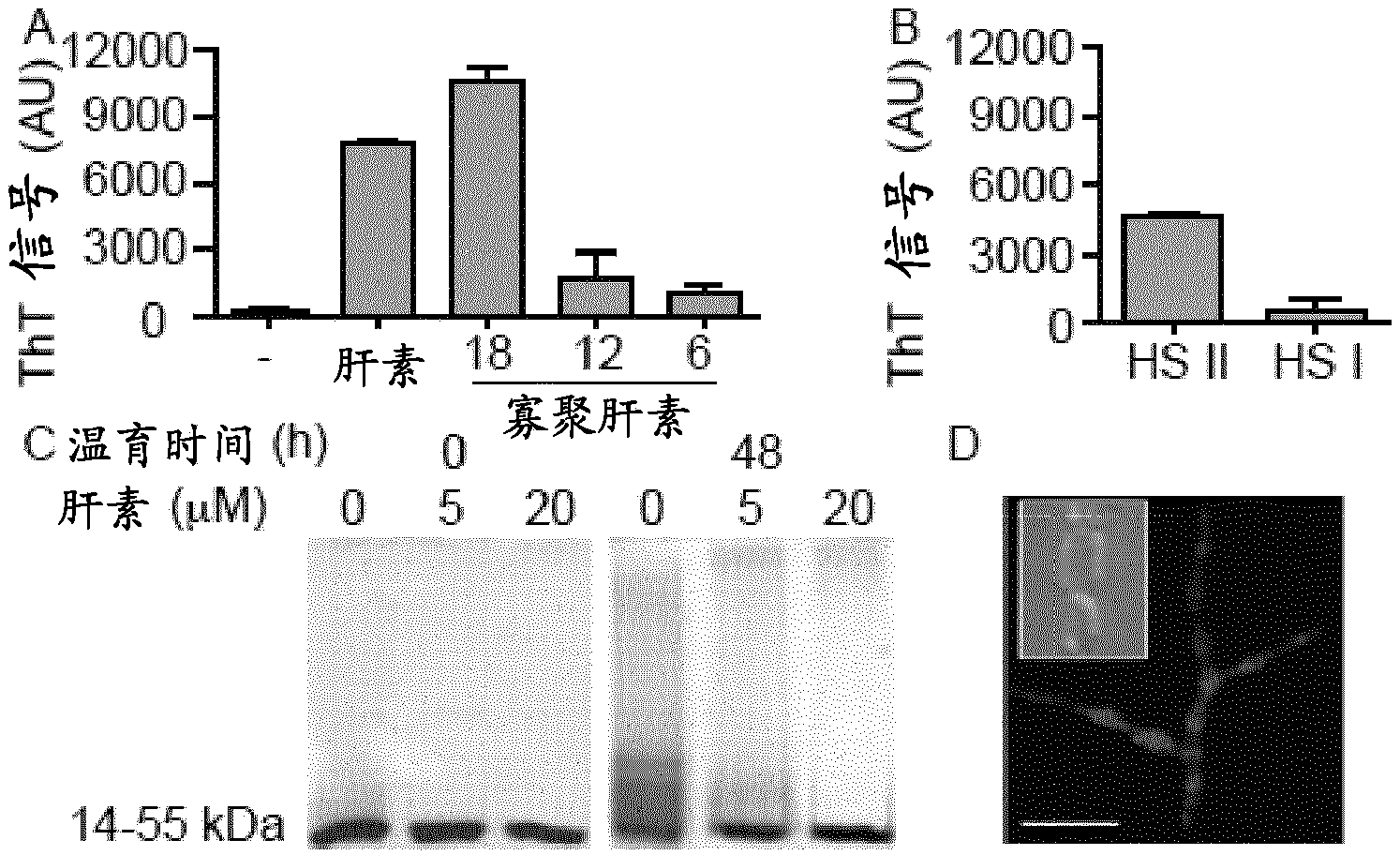
[0072] Recombinant wild-type TTR was expressed in bacteria and purified as described above (Furuya et al. (1991) Biochemistry 30:2415-2421). Purified proteins were dissolved in 20 mM phosphate-citrate buffer (pH 2.7, 3.5, 4.5 or 7.4) to a final concentration of 50 μM (for Thioflavin T fluorescence) or 500 μM (for non-denaturing PAGE). Heparin (3H-labeled or unlabeled) and HS were added to the TTR solution to the concentrations indicated by the reference symbols. Samples were incubated at 37°C for the stated times.
[0073] TTR peptide
[0074] Synthetic peptides corresponding to different TTR fragments were purchased from commercial sources or synthesize...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com