A kind of light hydrocarbon oil catalytic conversion method
A catalytic conversion method and technology for hydrocarbon oil, which are applied in the field of catalytic conversion of light hydrocarbon oil to produce low-carbon olefins, can solve the problems of difficulty in realizing high temperature reaction, high conversion rate of cracking reaction, consumption of a large amount of water vapor, etc., and reduce heat dissipation. The effect of total surface area, improving the yield of low-carbon olefins, and reducing energy consumption for heat dissipation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
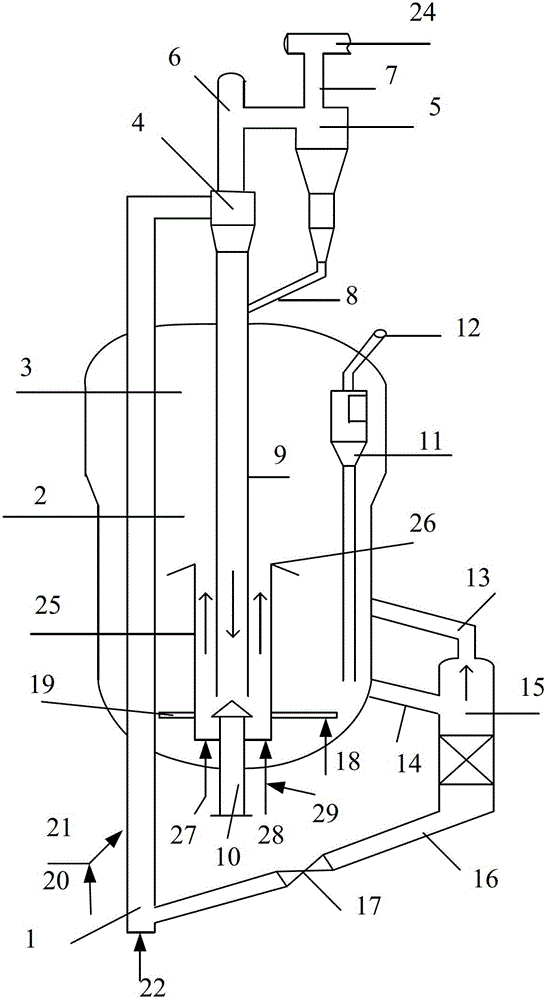
[0101] This embodiment 1 is tested according to the flow process of accompanying drawing, with straight-run naphtha as raw material, test is carried out on the medium-sized device of riser reactor, the raw material oil of preheating enters riser bottom, at reaction temperature 675 ℃, reaction The time is 2.0 seconds, the weight ratio of catalytic cracking catalyst to raw oil is 25, and the weight ratio of water vapor to raw oil is 0.5 to carry out the cracking reaction. The reaction product, water vapor and unborn catalyst enter the rough cyclone separator from the reactor outlet, The separated gas phase is further separated by the secondary cyclone separator, and the reaction oil gas is introduced into the separation system through the gas collection chamber to be cut according to the distillation range, so as to obtain dry gas, propylene, C4 and gasoline fractions, and the raw catalyst enters under the action of gravity To the regenerator, contact with air for regeneration. ...
Embodiment 2
[0104] The test device, test method and raw materials are the same as in Example 1, the difference is that the reaction oil gas is introduced into the separation system through the gas collection chamber and cut according to the distillation range, thereby obtaining dry gas, propylene, C4 and gasoline fractions, wherein C4 returns into the The reactor cracks further to ethylene and propylene. The spent catalyst enters the regenerator under the action of gravity and contacts with air for regeneration. The regenerated catalyst enters the degassing tank to remove the non-hydrocarbon gas impurities adsorbed and carried by the regenerated catalyst. The regenerated catalyst after stripping is returned to the riser reaction for recycling. The operating conditions and product distribution are listed in Table 2.
[0105] It can be seen from Table 2 that the yield of ethylene can reach 23.39% by weight, the yield of propylene can reach 24.71% by weight, and the ratio of propylene / ethy...
Embodiment 3
[0107] The test device, test method and raw materials are the same as in Example 1, the difference is that the reaction oil gas is introduced into the separation system through the gas collection chamber and cut according to the distillation range, thereby obtaining dry gas, propylene, C4 and gasoline fractions, wherein C4 returns into the The reactor cracks further to ethylene and propylene. C2-C3 alkanes enter the steam cracking unit, and react with water vapor at 830°C to separate and obtain the target products ethylene and propylene. The spent catalyst enters the regenerator under the action of gravity and contacts with air for regeneration. The regenerated catalyst enters the degassing tank to remove the non-hydrocarbon gas impurities adsorbed and carried by the regenerated catalyst. The regenerated catalyst after stripping is returned to the riser reaction for recycling. The operating conditions and product distribution are listed in Table 2.
[0108] It can be seen f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com