Preparation method of toothpaste-grade tin chloride
A stannous chloride, toothpaste-grade technology, applied in the field of preparation of toothpaste-grade stannous chloride, can solve the problems of low product purity, long synthesis process time, difficult use, etc., and achieve stable process, low cost, and less three wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
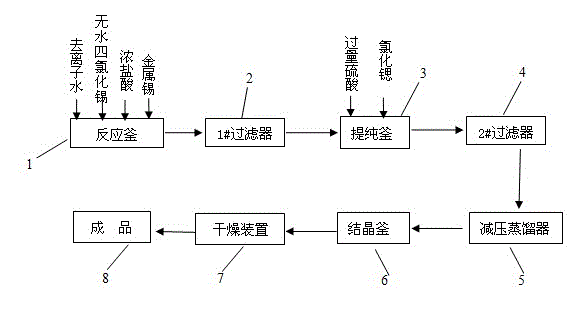
[0026] Such as figure 1 Shown, a kind of preparation method of toothpaste grade stannous chloride comprises the following steps:
[0027] (i) Synthesis reaction and primary filtration
[0028] Add deionized water into reaction kettle 1, then add concentrated hydrochloric acid, the amount of concentrated hydrochloric acid added is 3% to 8% of the total aqueous solution mass, then slowly add anhydrous tin tetrachloride dropwise, and finally put in metal tin, react The temperature is 110-120° C., and the reaction time is 1-3 hours. Wherein, the total aqueous solution quality refers to the sum of the aqueous solution contained in deionized water and concentrated hydrochloric acid; the molar ratio of deionized water, anhydrous tin tetrachloride and metallic tin is 4~8:1:1~1.2, the The mass concentration is 38%. The chemical reaction formula is:
[0029] Sn+SnCl 4 +4H 2 O→2SnCl 2 2H 2 o
[0030] The addition of concentrated hydrochloric acid effectively avoids the hyd...
Embodiment 1
[0039] 2.7kg of deionized water, 0.7kg of hydrochloric acid, 10kg of anhydrous tin tetrachloride, and 4.5kg of metal tin are added to the reactor successively, and the reaction temperature is 110°C, and the reaction time is 3h; after the reaction is completed, the metal solid impurities are filtered out; Add 42ml of sulfuric acid (10g / 100ml) to the filtrate, then add 42ml of strontium chloride (saturated solution), filter out solid impurities; take the clear solution and place it in a vacuum distiller for vacuum distillation with a vacuum of 0.01MPa and a temperature of 45 ℃; the distilled solution is introduced into the crystallization tank to stir and crystallize, the temperature is 0 ℃; after the white crystals are precipitated, the entire material system is placed in a centrifuge for solid-liquid separation, and the solid white crystals are put into a vacuum drying oven for drying , the vacuum degree is -0.05MPa, and the finished product is obtained. The weight of the obta...
Embodiment 2
[0041] 4.1kg of deionized water, 0.7kg of hydrochloric acid, 10kg of anhydrous tin tetrachloride, and 5.0kg of metal tin were added to the reactor successively, the reaction temperature was 115°C, and the reaction time was 2h; after the reaction was completed, the metal solid impurities were filtered out; Add 40ml of sulfuric acid (10g / 100ml) to the filtrate, then add 40ml of strontium chloride (saturated solution) to filter out solid impurities; take the clear solution and place it in a vacuum distiller for vacuum distillation with a vacuum of 0.03MPa and a temperature of 70 ℃; the distilled solution is introduced into the crystallization tank to stir and crystallize, the temperature is -5 ℃; after the white crystals are precipitated, the entire material system is placed in a centrifuge for solid-liquid separation, and the solid white crystals are put into a vacuum drying oven Drying, the vacuum degree is -0.08MPa, and the finished product is obtained. The weight of the obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com