Compound artemisinin multiphase lipidosome injection as well as preparation method thereof
A multiphase liposome and compound artemisinin technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of high equipment requirements and expensive equipment, and achieve high safety , good stability and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
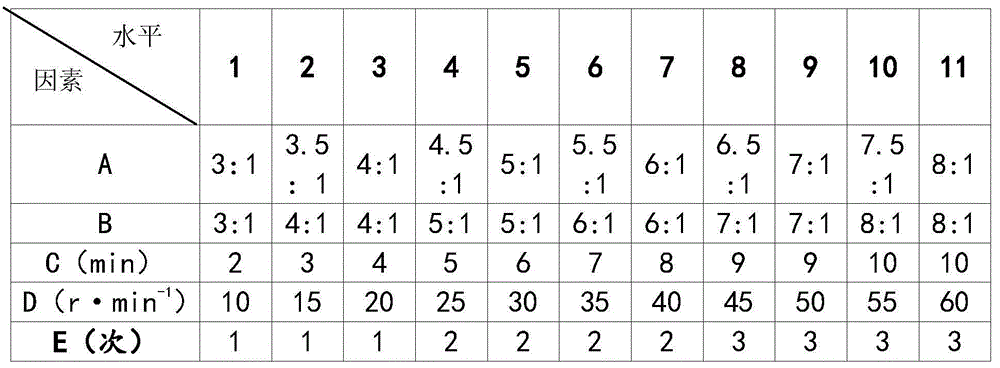
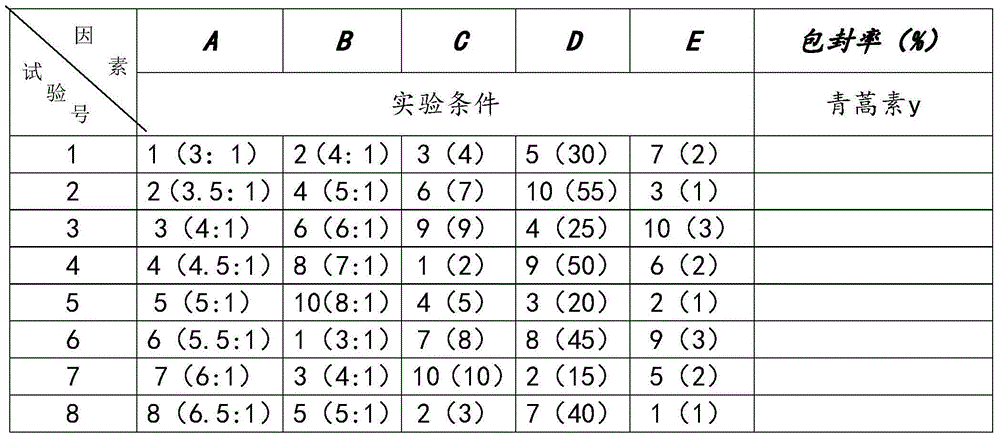
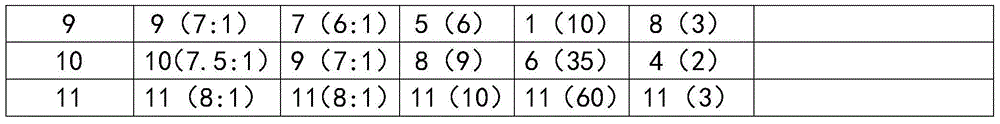
[0021] The compound artemisinin multiphase liposome injection includes medicinal ingredients and injection auxiliary ingredients, wherein the medicinal ingredients are made of artemisinin and triazamidine, and the injection auxiliary ingredients are lecithin, cholesterol, Tween -80, phosphate buffer and absolute ethanol.
[0022] The components are calculated in parts by weight: 1 part of lecithin, 0.5 parts of cholesterol, 5 parts of Tween-80, 5 parts of artemisinin, 5 parts of triazamidine, 10 parts of absolute ethanol, and 50 parts of phosphate buffer.
Embodiment 2
[0024] The compound artemisinin multiphase liposome injection includes medicinal ingredients and injection auxiliary ingredients, wherein the medicinal ingredients are made of artemisinin and triazamidine, and the injection auxiliary ingredients are lecithin, cholesterol, Tween -80, phosphate buffer and absolute ethanol.
[0025] The components are calculated in parts by weight: 10 parts of lecithin, 5 parts of cholesterol, 3 parts of Tween-803, 5 parts of artemisinin, 4 parts of triazamidine, 8 parts of absolute ethanol, and 45 parts of phosphate buffer.
Embodiment 3
[0027] The compound artemisinin multiphase liposome injection includes medicinal ingredients and injection auxiliary ingredients, wherein the medicinal ingredients are made of artemisinin and triazamidine, and the injection auxiliary ingredients are lecithin, cholesterol, Tween -80, phosphate buffer and absolute ethanol.
[0028] The components are calculated in parts by weight: 30 parts of lecithin, 10 parts of cholesterol, 0.5 parts of Tween-800, 1 part of artemisinin, 3 parts of triazamidine, 7 parts of absolute ethanol, and 55 parts of phosphate buffer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com