Pyrimidotricyclic compounds or pyrimidotetracyclic compounds, pharmaceutical composition and applications thereof
A compound and tetracyclic technology, applied in the field of pyrimidotricyclic or pyrimidotetracyclic compounds and their pharmaceutical compositions, can solve poor selectivity, wild-type cytotoxic side effects, and can not solve the clinical pressure of drug resistance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
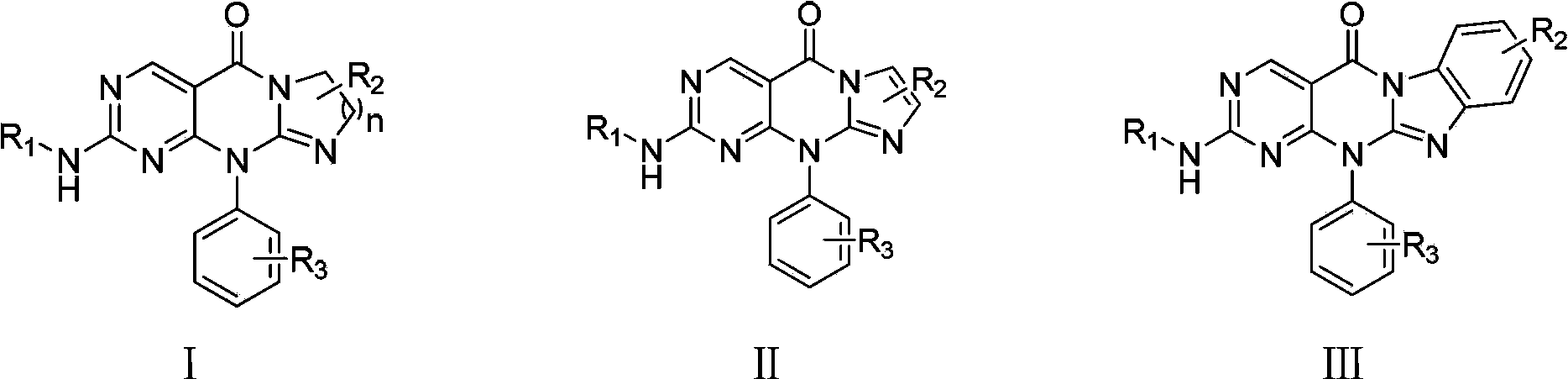
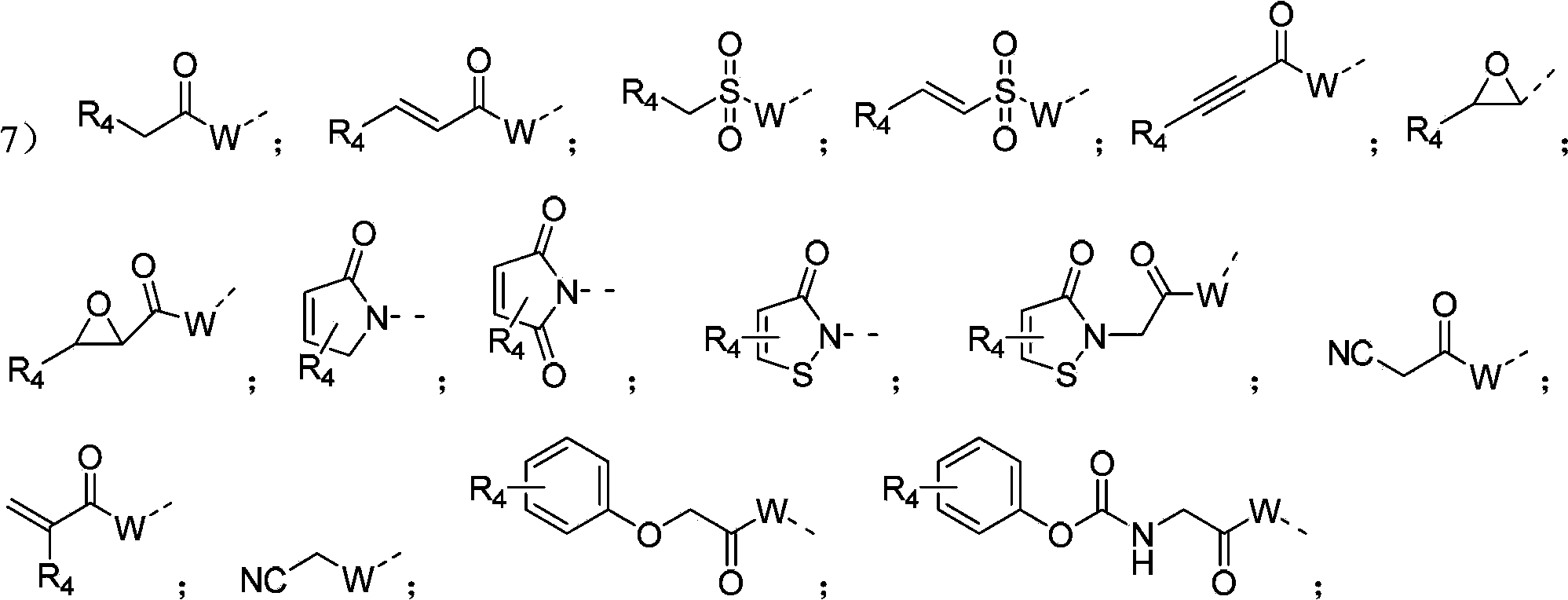
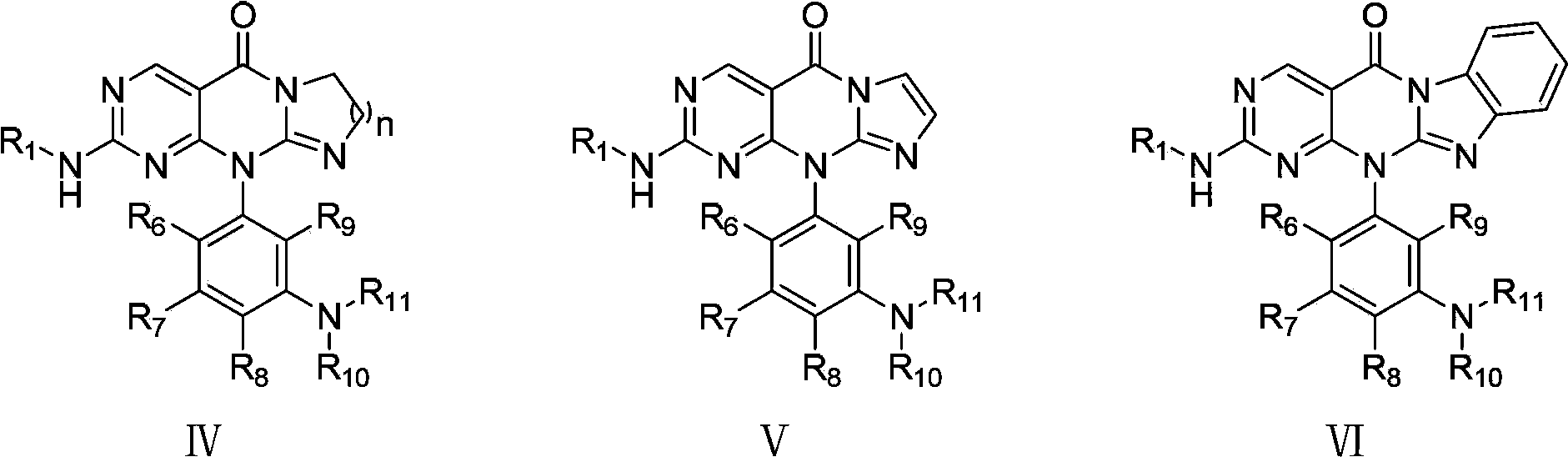
Image
Examples
Embodiment 1
[0161] N-(3-(2-((2-methoxy-4-(4-methyl-1-piperazinyl)phenyl)amino)-5-oxo-7,8-dihydroimidazo[ 1,2-a]pyrimido[4,5-d]pyrimidin-10(5hydro)-yl)phenyl)acrylamide (XTF-01)
[0162]
[0163] Step 1. 4-(3-tert-butoxycarbonylaminoaniline)-2-chloropyrimidine-5-ethyl carbonate (1)
[0164]
[0165] 2,4-dichloro-5-pyrimidinecarboxylic acid ethyl ester (22.1g, 100mmol, 1.0eq), N-Boc m-phenylenediamine (20.8g, 1.0eq), DIPEA (17.4ml, 1.0eq) at room temperature Add in 500ml of acetonitrile, heat to reflux and stir to react for 2 hours, stop heating until the temperature of the system drops to room temperature, solid precipitates, and filter under reduced pressure to obtain 35.3g (90%) of white solid.
[0166] 1 H NMR (400MHz, CDCl 3 )δ10.44(s,1H,),8.81(s,1H),7.79(s,1H),7.36(d,J=7.6Hz,1H),7.31(d,J=8.0Hz,1H),7.18 (d, J=8.0Hz, 1H), 6.55(s, 1H), 4.44(q, J=7.2Hz, 2H), 1.52(s, 9H), 1.42(t, J=7.2Hz, 3H).
[0167] Step 2. 4-(3-tert-butoxycarbonylaminoaniline)-2-chloro-5-pyrimidinecarboxylic...
Embodiment 2
[0186] N-(3-(2-((4-(4-acetyl-1-piperazinyl)-2-methoxyphenyl)amino)-5-oxo-7,8-dihydroimidazo[ 1,2-a]pyrimido[4,5-d]pyrimidine-10(5hydrogen)-substituted)phenyl)acrylamide (XTF-02)
[0187]
[0188] The synthetic method is as embodiment 1
[0189] 1 H NMR (400MHz, Acetic-d 4 )δ9.00(s,1H),8.13(d,J=7.6Hz,1H),7.96(s,1H),7.62(t,J=8.0Hz,1H),7.28-7.34(m,2H), 6.71(s,1H),6.46-6.48(m,1H),6.26(brs,1H),5.84(dd,J=3.6,8.0Hz,1H),4.43(t,J=9.2Hz,2H),4.09 (t,J=9.2Hz,2H),3.84(m,5H),3.74(m,2H),3.18-3.24(m,4H),2.21(s,3H).LCMS(ESI):m / z 582.2 [M+H] +
Embodiment 3
[0191] N-3-(2-((4-(4-(dimethylamino)-1-piperazine)-2methoxyphenyl)amino)-5-oxo-7,8-dihydroimidazo[ 1,2-a]pyrimido[4,5-d]pyrimidine-10(5hydrogen)-substituted)phenyl)acrylamide (XTF-03)
[0192]
[0193] The synthesis method is as in Example 1.
[0194] 1 H NMR (400MHz, Acetic-d 4 )δ9.03(s,1H),8.14(s,1H),8.03(d,J=7.2Hz,1H),7.64(t,J=8.0Hz,1H),7.43(d,J=8.0Hz, 1H), 7.33(d, J=7.6Hz, 1H), 7.01(s, 1H), 6.60-6.50(m, 2H), 6.46(d, J=16.4Hz, 1H), 5.84(d, J=10.8 Hz,1H),4.45(t,J=8.8Hz,2H),4.11(t,J=8.8Hz,2H),3.87(s,3H),3.81(m,2H),3.70(m,1H), 3.29(t,J=11.2Hz,2H),2.93(s,6H),2.40-2.15(m,4H).LCMS(ESI):m / z 582.2[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com