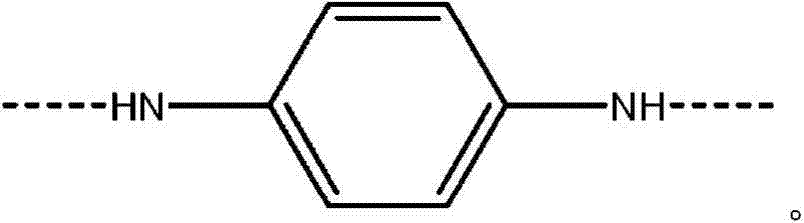
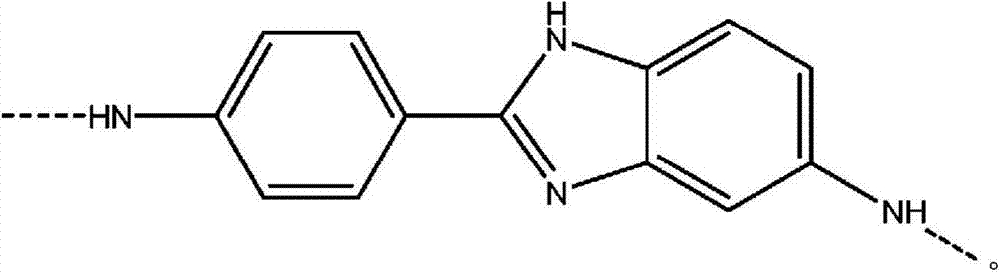
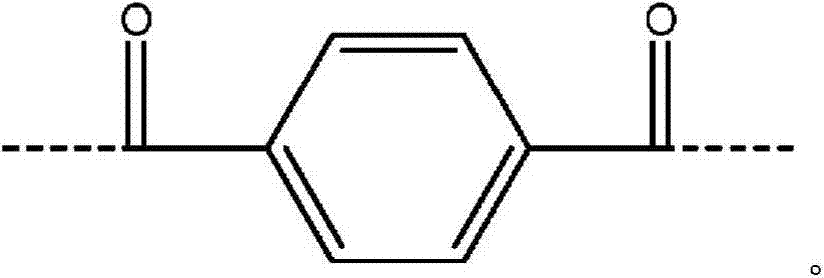
Process for forming an aramid copolymer
A technology of polymers and oligomers, which is applied in the field of preparing aromatic polyamide polymers, and can solve problems such as not allowing the preparation of aromatic polyamide polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0071] Charge the FM130D Littleford reactor containing 5.38% CaCl 2 31.82 kg of NMP solvent ("c" value 5.38). Then 455 g of PPD ("y" value of 30) and 2180 g of DAPBI ("b" value of 70) were charged. The process was then cooled to 7°C. Three additions of terephthaloyl chloride were made: 998g, 997g and 834g. After the first addition, the mixture was cooled to 10°C and after the second addition, the mixture was cooled to 10°C. At the end of the reaction, the reactor was inspected for sediment and no large clumps of DAPBI were observed on the walls. The product of (b×c) is equal to 377.
[0072] The solids in the reaction were 12% on a polymer basis and 14.7% on a total monomer basis. The final intrinsic viscosity was 7.5. The final polymer had a polydispersity of about 1.5 and a <3000 MW oligomer content of about 0.45%.
example 2
[0077] Charge the FM130D Littleford reactor containing 5.91% CaCl 2 31.36 kg of NMP solvent ("c" value 5.91). Then 493 g of PPD ("y" value of 30) and 2382 g of DAPBI ("b" value of 70) were charged. The process was then cooled to 8°C. Three terephthaloyl chloride additions were made: 772g, 773g and 1538g. After the first addition, the mixture was cooled to 1OC and after the second addition, the mixture was cooled to 11°C. At the end of the reaction, the reactor was inspected for sediment and no large clumps of DAPBI were observed on the walls. The product of (b×c) is equal to 414.
[0078] The solids in the reaction were 13% on a polymer basis and 16.0% on a total monomer basis. The final intrinsic viscosity was 7.1.
example 3
[0080] The polymer was prepared by the method of Example 2, and the weight average molecular weight and number average molecular weight were obtained. The polymer has a PDI of less than 1.5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com