Porphyrin compound containing acid anhydride bond symmetrical molecular tweezers series and preparation method thereof
A technology of porphyrin compounds and symmetrical molecules, which is applied in the field of a series of metal porphyrin compounds and their preparation, and can solve the problems of incomplete detection performance and few types, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
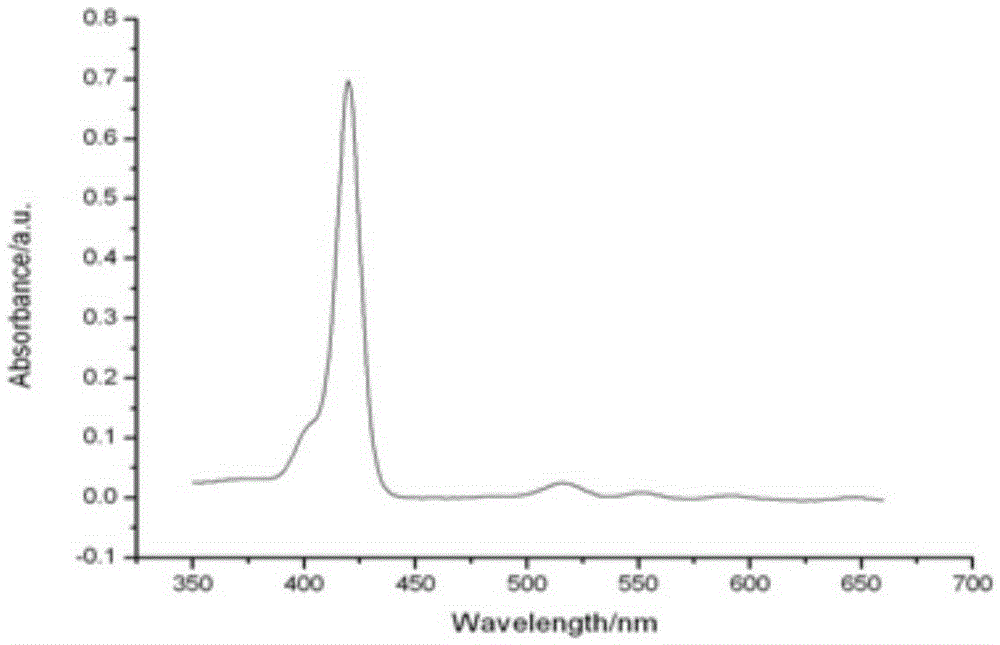
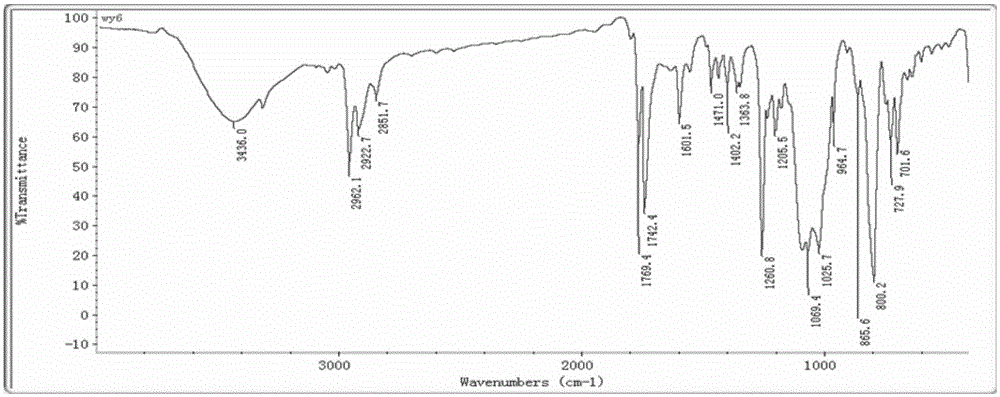
[0045] The molecular tweezers series porphyrin compound containing acid anhydride bond symmetry, the preparation method thereof, and the application for detecting hexanal in the present invention will be further described in detail below in conjunction with specific examples.
[0046] 1. A molecular clamp series porphyrin compound containing acid anhydride bond symmetry.
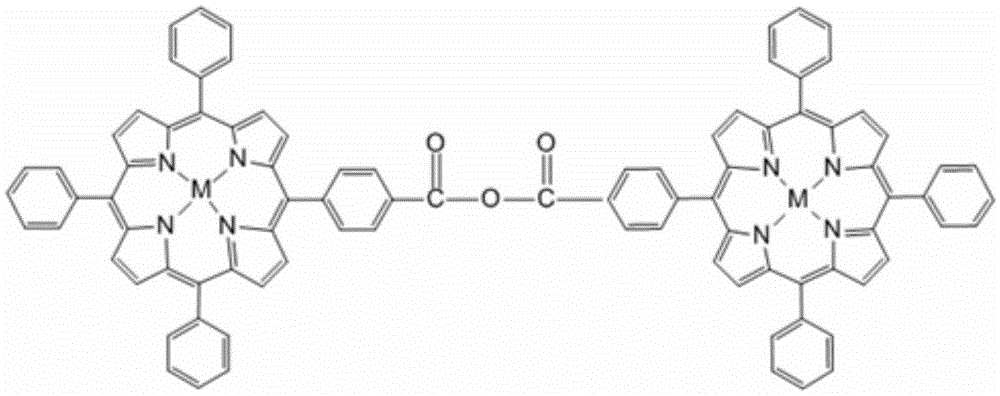
[0047] The structural formula of the porphyrin compound is as figure 1 Shown:
[0048]
[0049] Among them, M is 2H, and the molecular formula is C 90 h 58 N 8 o 3 ; Referred to as free base porphyrin P.
[0050] Said molecular tweezers series metalloporphyrin compound containing acid anhydride bond symmetry: M is Zn, MnCl, Cu, Ni or Co.
[0051] When M is Zn, the molecular formula is C 90 h 54 N 8 o 3 Zn 2 ; Referred to as zinc porphyrin ZnP;
[0052] When M is MnCl, the molecular formula is C 90 h 54 N 8 o 3 Cl 2 mn 2 ; Abbreviated as manganese porphyrin MnClP;
[0053] When M is Cu, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com