Oxidative desulphurization and brimstone recycling method
A technology for sulfur recovery and oxidative desulfurization, applied in chemical instruments and methods, separation methods, sulfur preparation/purification, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
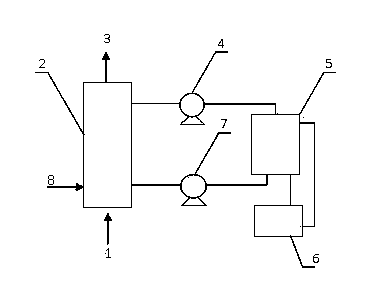
[0038] Removal of H 2 The reaction conditions at S are as follows: In the absorber: the temperature is 50°C, the reaction pressure is 0.6MPa, and the 2 The volume ratio of the hourly flow rate of S gas to complex iron desulfurization liquid is 15:1; in the mixer: the volume ratio of desulfurization liquid to oil phase (catalytically cracked diesel oil with a distillation range of 240~260°C) is 1:10, and the reaction The temperature is 60°C, and the reaction time is 50 minutes; in the solid-liquid separator: the temperature for cooling and separating sulfur is 10°C.
[0039]Composition of hydrogen sulfide-containing gas (volume): hydrogen sulfide content is 0.65%, SO 2 is 0.01%, COS is 0.01%; the concentration of Fe ions in the complex iron desulfurization solution is 0.06mol / L; after oxidative desulfurization of the desulfurization solution, the H in the gas is purified 2 S concentration is 15mg / m 3 , The purity of recovered sulfur is 98%.
Embodiment 2
[0041] Removal of H 2 The reaction conditions at S are as follows: In the absorber: the temperature is 60°C, the reaction pressure is 0.3MPa, and the 2 The volume ratio of the hourly flow rate of S gas to complex iron desulfurization liquid is 30:1; in the mixer: the volume ratio of desulfurization liquid to oil phase (catalytically cracked diesel oil with a distillation range of 260~300°C) is 1:5, and the reaction The temperature is 80°C, and the reaction time is 20 minutes; in the solid-liquid separator: the temperature for cooling and separating sulfur is 5°C.
[0042] Composition of hydrogen sulfide-containing gas (volume): hydrogen sulfide content is 0.18%, SO 2 is 0.1%, COS is 0.03%; the concentration of Fe ions in the complex iron desulfurization solution is 0.08mol / L; after oxidative desulfurization of the desulfurization solution, the H in the gas is purified 2 S concentration is 8mg / m 3 , The purity of recovered sulfur is 98.7%.
Embodiment 3
[0044] Removal of H 2 The reaction conditions at S are as follows: In the absorber: the temperature is 80°C, the reaction pressure is 0.1MPa, and the 2 The volume ratio of the hourly flow rate of S gas to complex iron desulfurization liquid is 1:8; in the mixer: the volume ratio of desulfurization liquid to oil phase (catalytically cracked diesel oil with a distillation range of 300~330°C) is 1:1, and the reaction The temperature is 100°C, and the reaction time is 10 minutes; in the solid-liquid separator: the temperature for cooling and separating sulfur is 15°C.
[0045] Composition of hydrogen sulfide-containing gas (volume): hydrogen sulfide content is 1.05%, SO 2 is 0.36%, COS is 0.27%; the concentration of Fe ions in complex iron desulfurization solution is 0.1mol / L; after oxidative desulfurization of desulfurization solution, the H in the gas is purified 2 S concentration is 10mg / m 3 , The purity of recovered sulfur is 98.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com