Preparation method of 3-methyl glutaric acid compound
A technology of methylglutaric acid and methylglutaramide, applied in the field of medicine and chemical industry, can solve the problems of harsh reaction conditions, low yield, complicated operation, etc., and achieves easily available price, high product yield, and reaction operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0023] The present invention is described further below by embodiment, so that better understanding of the present invention. The protection content of the present invention is not limited to the following examples. Without departing from the spirit and scope of the inventive concept, changes and advantages conceivable by those skilled in the art are all included in the present invention, and the appended claims are the protection scope. The process, conditions, reagents, experimental methods, etc. for implementing the present invention are general knowledge and common knowledge in the art except for the content specifically mentioned below, and the present invention has no special limitation content.
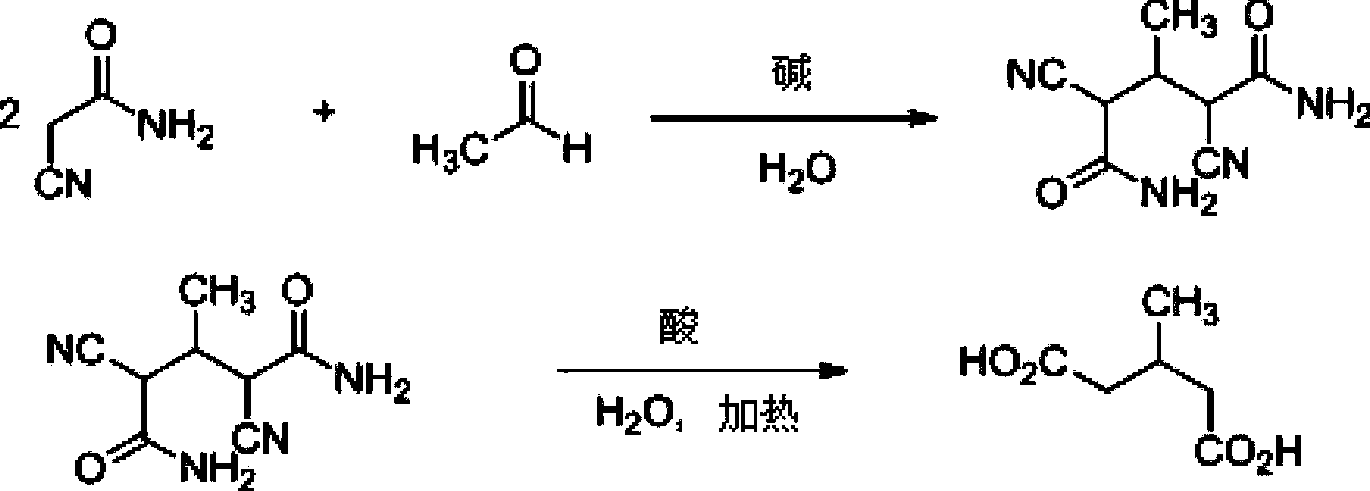
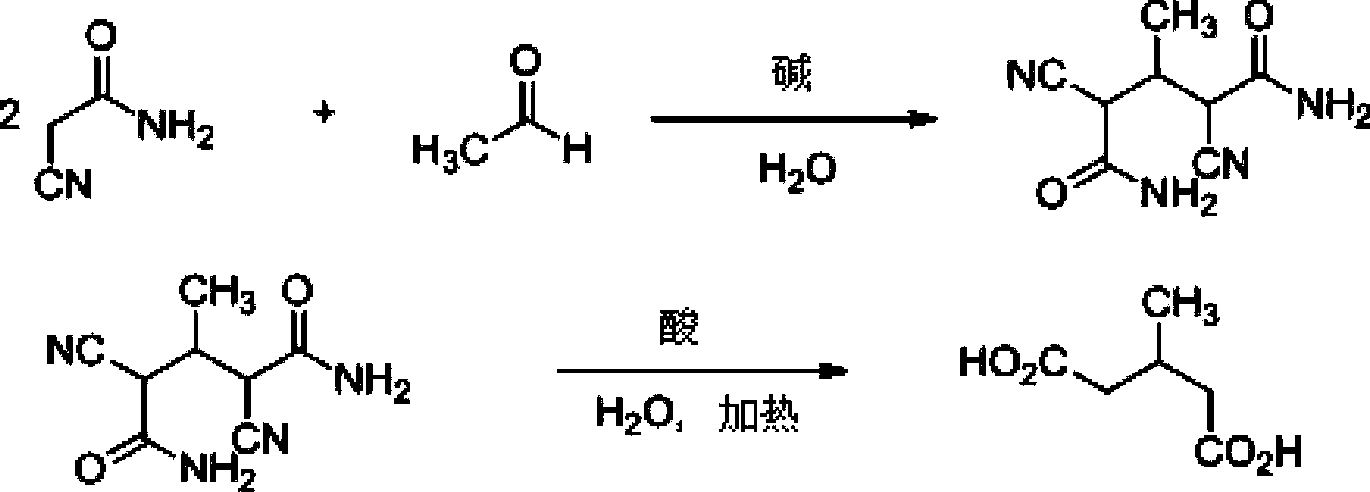
[0024] The preparation method of the present invention uses cyanoacetamide and acetaldehyde as raw materials, and under basic catalytic conditions, reacts to obtain α, α'-dicyano-β-methylglutaramide condensate; α, α'- Dicyano-β-methylglutaramide condensate is mixed with water,...
Embodiment 1
[0033] (1) Synthesis of α, α'-dicyano-β-methylglutaramide condensate
[0034] Add 16.8g cyanoacetamide, 13.6g acetaldehyde solution containing 32.3% acetaldehyde, 63ml water at one time in a 100ml three-necked flask, stir mechanically, add 0.5ml morpholine dropwise at room temperature (20°C), the solution becomes clear, and then It became white and turbid again, and gradually became viscous. Heat preservation reaction for 12 hours. Filter, wash with ice water, and dry to obtain 18g of α,α'-dicyano-β-methylglutaramide condensate, with a yield of 93% and a melting point of 154-159°C.
[0035] (2) Synthesis of 3-methylglutaric acid
[0036] In the 100ml reaction bottle, drop into 8.8g α, α'-dicyano-β-methyl glutaramide condensate, 14ml water, drop 20g98% concentrated sulfuric acid below 20 ℃. The reaction was carried out at 20° C. for 2 hours, and then the temperature was slowly raised to 80° C., and the reaction solution became clear. Slowly raise the temperature to 130°C, a...
Embodiment 2
[0038] The preparation process of this embodiment is basically the same as that of Example 1.
[0039] (1) Synthesis of α, α'-dicyano-β-methylglutaramide condensate
[0040] Add 18.48g cyanoacetamide, 13.6g acetaldehyde solution containing 32.3% acetaldehyde, 70ml water at one time to a 100ml three-necked flask, stir mechanically, add 0.6ml piperidine dropwise at room temperature (20°C), the solution becomes clear, and then It became white and turbid again, and gradually became viscous. Insulated reaction for 14 hours. Filter, wash with ice water, and dry to obtain 17.3g of α,α'-dicyano-β-methylglutaramide condensate, with a yield of 89% and a melting point of 154-159°C.
[0041] (2) Synthesis of 3-methylglutaric acid
[0042]8.8 g of α, α'-dicyano-β-methylglutaramide condensate was dropped into a 100 ml reaction bottle, and 50 g of 31% concentrated hydrochloric acid solution was added dropwise at 20-25°C. The reaction was carried out at 20° C. for 2 hours, and then the te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com