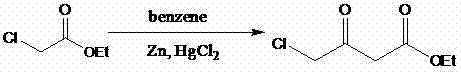Preparation method of 4-chloracetyl ethyl acetate
A technology of ethyl chloroacetoacetate and esterification, which is applied in the preparation of acyl halides, carboxylic acid halides, organic chemistry, etc., can solve the problems of high production costs and low yields, and achieve increased distillation yields and reduced costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 84g of diketene, 220g of dichloromethane, and 0.4g of anhydrous copper sulfate (0.48%) into the reaction kettle, start stirring, cool down to -10°C, and introduce 44g of chlorine gas. After aeration, add 55g of ethanol dropwise. After the reaction is completed The dichloromethane was distilled off to obtain the crude product of ethyl 4-chloroacetoacetate (the content of ethyl 2-chloroacetoacetate was 0.42%). After rectification, 156.3 g of the product was obtained, with a yield of 95.04%.
Embodiment 2
[0025] Add 84g of diketene, 220g of dichloromethane, and 0.84g of anhydrous copper sulfate (1%) into the reaction kettle, start stirring, cool down to -10°C, and introduce 44g of chlorine gas. After aeration, add 55g of ethanol dropwise. After the reaction is completed The dichloromethane was distilled off to obtain the crude product of ethyl 4-chloroacetoacetate (the content of ethyl 2-chloroacetoacetate was 0.49%). After rectification, 155.5 g of the product was obtained, with a yield of 94.55%.
Embodiment 3
[0027] Add 84g of diketene, 220g of dichloromethane, and 0.08g (0.1%) of anhydrous copper sulfate to the reaction kettle, start stirring, cool down to -10°C, and introduce 44g of chlorine gas. After the aeration is completed, add 55g of ethanol dropwise. The dichloromethane was distilled off to obtain the crude product of ethyl 4-chloroacetoacetate (the content of ethyl 2-chloroacetoacetate was 0.31%). After rectification, 158g of the product was obtained, with a yield of 96.08%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com