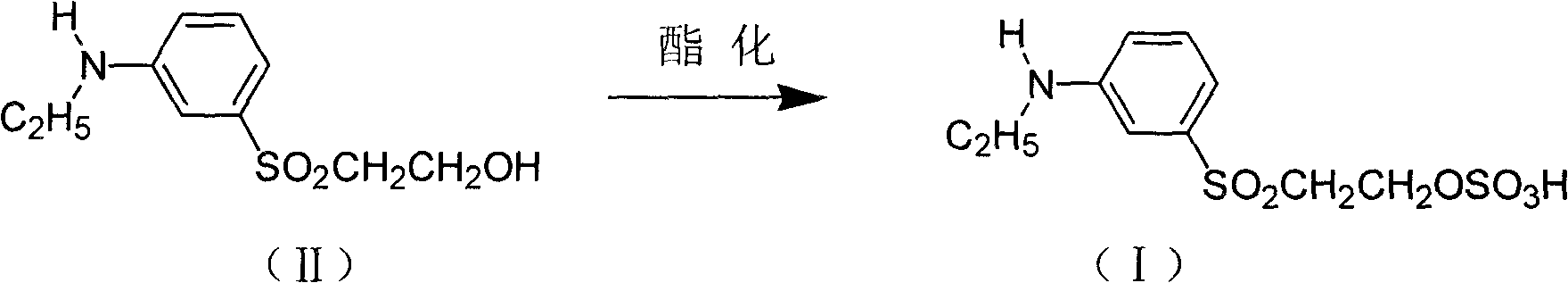
Method for synthesizing 3-(beta-sulfate ethyl sulfuryl)-N-ethyl aniline
A technology of sulfate ethyl sulfone and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of difficulty in realizing industrialization, high production cost, difficult recycling and the like, and achieves the reduction of waste water. The effect of producing, reducing dosage and saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 250mL three-necked flask with stirring and reflux separator, add 150mL toluene, 0.025mol dibenzyl dodecylamine, 0.05mol 3-(β-hydroxyethylsulfone)-N-ethylaniline and 0.1mol 98% sulfuric acid, stir and mix evenly, heat up to reflux for 8 hours, high performance liquid chromatography (HPLC) detects that the content of 3-(γ-hydroxyethylsulfone)-N-ethylaniline 3 Aqueous solution, fully stirred and reacted for 2h, allowed to stand for stratification, the upper layer solution was reserved for the next application, the lower layer was 3-(β-sulfate ethyl sulfone group)-N-ethylaniline aqueous solution, dried to obtain a solid product, obtained The HPLC purity of the product is >95%, the diazotization content is >80%, and the yield is >85%.
Embodiment 2
[0025] Add 0.05mol 3-(β-hydroxyethylsulfone)-N-ethylaniline and 0.075mol 80% sulfuric acid to the upper layer solution of Example 1 above, stir and mix evenly, heat up to reflux for 9h, and perform high performance liquid chromatography (HPLC) detection of 3-(β-hydroxyethyl sulfone group)-N-ethylaniline content3 Aqueous solution, fully stirred and reacted for 2-3 hours, standing for stratification, the upper layer solution is reserved for the next application, the lower layer is 3-(β-sulfate ethyl sulfone group)-N-ethylaniline aqueous solution, HPLC purity > 95% , diazotization content > 80%, yield > 90%.
Embodiment 3
[0027] Xylene was used to replace the toluene in Example 1, and other conditions were the same, the HPLC purity of the obtained product was >92%, and the yield was >85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com