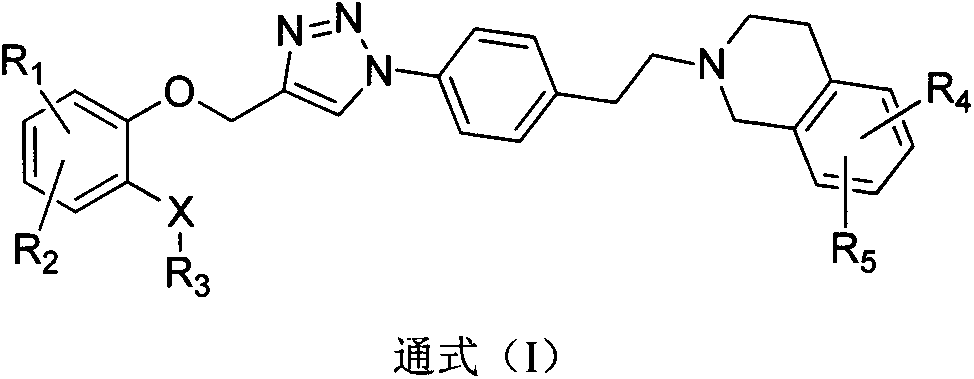
Triazole phenethyl tetrahydro naphthalene compound and preparation method and application thereof
A technology of dihydroisoquinolines and compounds, applied in drug combination, organic chemistry, pharmaceutical formulations, etc., can solve problems such as limited application, major cardiovascular and other side effects, and changes in plasma pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Preparation of 2-(4-azidophenethyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline (2)
[0075] Compound 4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)aniline (3.72g, 12mmol) was dissolved in acetic acid and water at room temperature (12ml / 12ml) mixed solution. The temperature was cooled to -5°C in an ice-salt bath, and sodium nitrite (1.2 g, 17 mmol) was carefully added three times, the reaction solution was wine red, and kept stirring at 0-5°C for 30 min. The internal temperature was controlled at -5-5°C, and sodium azide (1.2 g, 18 mmol) was carefully added several times. During the addition process, the temperature rose violently, and a large number of bubbles were generated. After adding sodium azide, the reaction was continued at 0°C for 50 min. Add water (60ml) to the reaction solution for dilution, carefully add sodium carbonate to the reaction solution under stirring to adjust the pH to 7-8, extract with ethyl acetate (60ml×2), wash with saturated b...
Embodiment 2
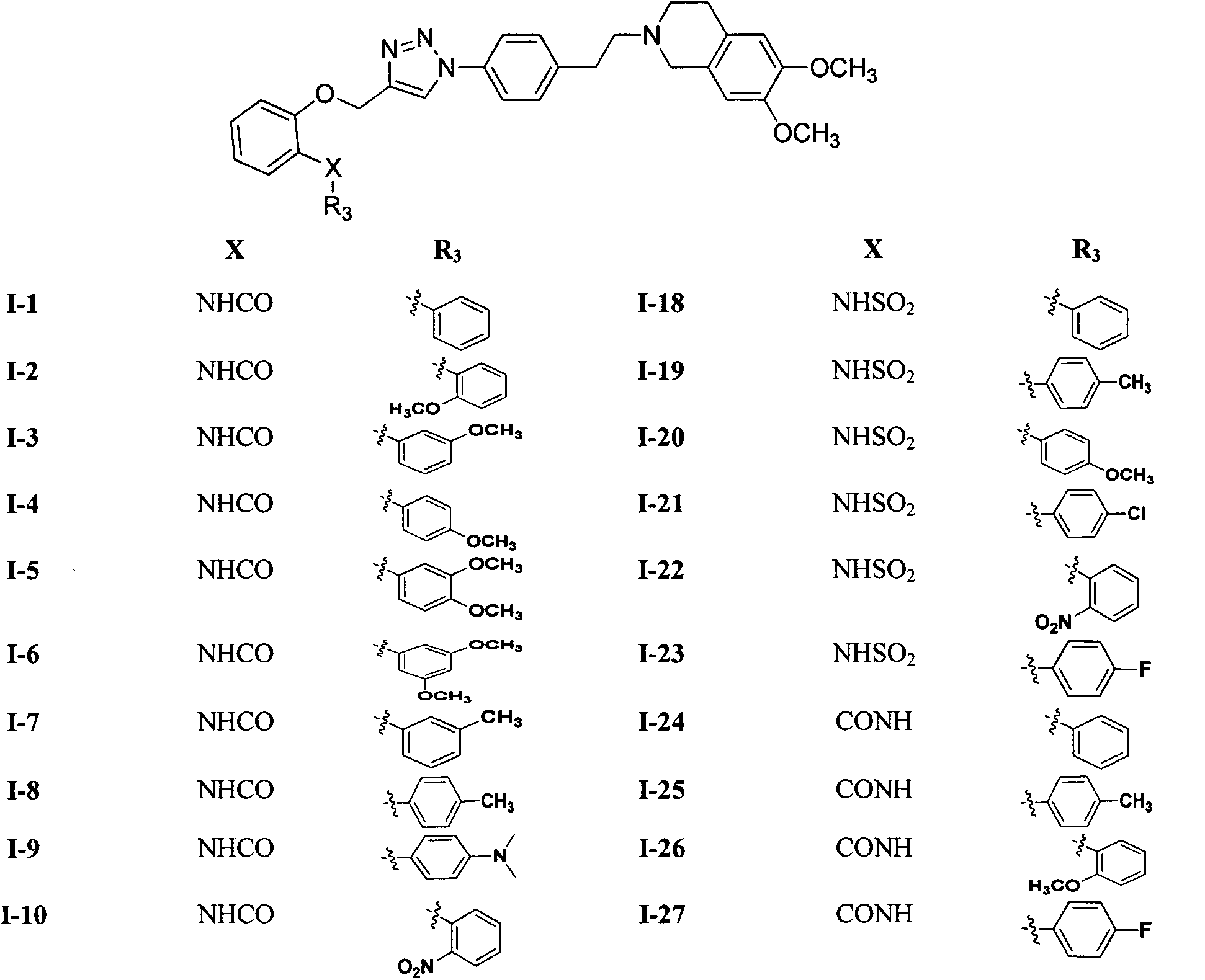
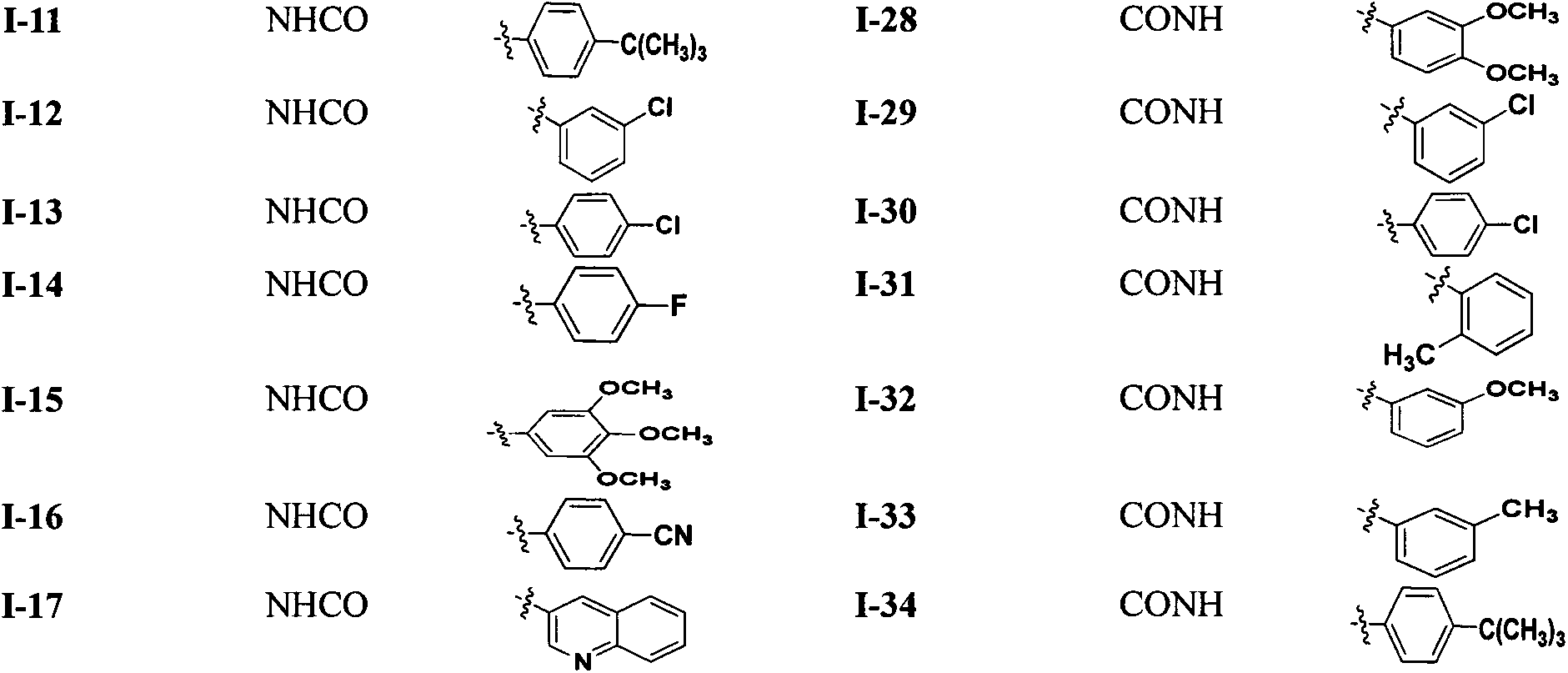
[0079] N-(2-((1-(4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2-(1H)-yl)-ethyl)phenyl) -1H-1,2,3-triazol-4-yl)methoxy)phenyl)benzamide (I-1) preparation
[0080] Compound 2 (3.34g, 1mmol) and N-(2-(prop-2-yn-1-yloxy)phenyl)benzamide (0.25g, 1mmol) were dissolved in 75% methanol (40ml), and were added Sodium ascorbate (30mg) and copper sulfate (10mg), the reaction solution was stirred at room temperature for 24h, the precipitated solid was suction filtered, washed with methanol, and dried to obtain 0.52g of white solid, yield 80.2%, mp: 104-105°C.
[0081] 1 H NMR (300MHz, DMSO-d 6 )δ: 9.30 (s, 1H, CONH), 8.82 (s, 1H, NCH=C), 7.86 (d, J=6.9Hz, 1H, ArH), 7.72 (d, J=8.3Hz, 2H, ArH) , 7.56(d, J=8.4Hz, 2H, ArH), 7.48-7.45(m, 3H, ArH), 7.35(d, J=8.1Hz, 1H, ArH), 7.18(dd, J=7.0, 7.5Hz , 1H, ArH), 7.04-7.00 (m, 2H, ArH), 6.65, 6.62 (2s, 2H, ArH), 5.33 (s, 2H, OCH 2 ), 3.80 (s, 3H, OCH 3 ), 3.73-3.69 (m, 9H, 3OCH 3 ), 3.54(s, 2H, ArCH 2 N), 2.90-2.70 (m, 8H, 4CH 2 ); 13 C NMR (...
Embodiment 3
[0083] N-(2-((1-(4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2-(1H)-yl)-ethyl)phenyl) -1H-1,2,3-triazol-4-yl)methoxy)phenyl)-2-methoxybenzamide (I-2) preparation
[0084] Referring to the preparation method of I-1 in Example 2, by compound 2 (1mmol) and 3,4-dimethoxy-N-(2-(prop-2-yn-1-yloxy)phenyl)benzyl Compound I-2 was prepared from amide (1 mmol) as a white solid with a yield of 64.5%; mp: 134-136°C.
[0085] 1 H NMR (300MHz, DMSO-d 6 )δ: 10.53 (s, 1H, CONH), 9.01 (s, 1H, NCH=C), 8.55 (d, J=7.1Hz, 1H, ArH), 8.08 (d, J=6.5Hz, 1H, ArH) , 7.81(d, J=8.3Hz, 2H, ArH), 7.54-7.48(m, 3H, ArH), 7.36(d, J=7.9Hz, 1H, ArH), 7.16-7.09(m, 3H, ArH) , 7.01 (dd, J=7.7, 7.5Hz, 1H, ArH), 6.65, 6.63 (2s, 2H, ArH), 5.37 (s, 2H, OCH 2 ), 3.69(s, 6H, 2OCH 3 ), 3.53 (s, 5H, OCH 3 , ArCH 2 N), 2.94-2.71 (m, 8H, 4CH 2 ); 13 C NMR (300MHz, DMSO-d 6 )δ:162.08,157.02,147.14,146.88,143.19,141.64,134.55,133.57,131.39,130.08,128.03,126.56,125.87,123.61,123.54,121.10,120.87,120.11,119.53,112.46,11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com