A kind of method for preparing ethylene glycol
A technology of ethylene glycol and catalyst, which is applied in the field of catalyst and its application, can solve the problems of poor cycle stability of catalyst, low selectivity of ethylene glycol, cumbersome preparation process, etc., and achieve high atom economy and high product yield and selectivity, preparation of simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of metal catalysts Raney Ni, Raney Co, Raney Fe, Raney Cu:
[0020] Raney Ni: Add a Ni-Al alloy with a Ni / Al ratio of 1:1 to a 20% sodium hydroxide solution at 50°C within 20 minutes, then heat to 80°C, and keep it at 80°C for 120 minutes, and keep stirring during the whole process . After the reaction, cool down, filter, wash the filter cake with ethanol three times and then wash with distilled water to PH=7, and finally store the prepared Raney nickel in absolute ethanol for later use (see patent: Prepared with Raney nickel as catalyst The method of 3-amino-4-methoxyacetanilide, CN101880242).
[0021] In the same way, Co / Al alloy, or Fe / Al alloy, or Cu / Al alloy is activated to produce Raney Co, Raney Fe, and Raney Cu in sequence.
[0022] The amorphous Raney nickel catalyst is a commercial catalyst purchased directly from Anshan Zhongli Catalyst Factory, model ZL-N311.
Embodiment 2
[0024] Catalytic conversion experiment: Add 10.0g of polyol, 0.3g of catalyst A, 0.1g of catalyst B and 100ml of water into a 200ml reaction kettle, replace the gas with hydrogen for three times, fill with hydrogen to 5MPa, heat up to 240°C for 30min . After the reaction, cool down to room temperature, take the centrifuged supernatant, separate it on a high-performance liquid chromatography calcium-type ion-exchange column and detect it with a differential refraction detector. In the product yield, only the target products ethylene glycol, propylene glycol and hexahydric alcohols (including sorbitol and mannitol) are calculated, and other liquid products include butylene glycol, ethanol, unknown components, and gas products (CO 2 , CH 4 , C 2 h 6 etc.) The yield was not calculated.
Embodiment 3
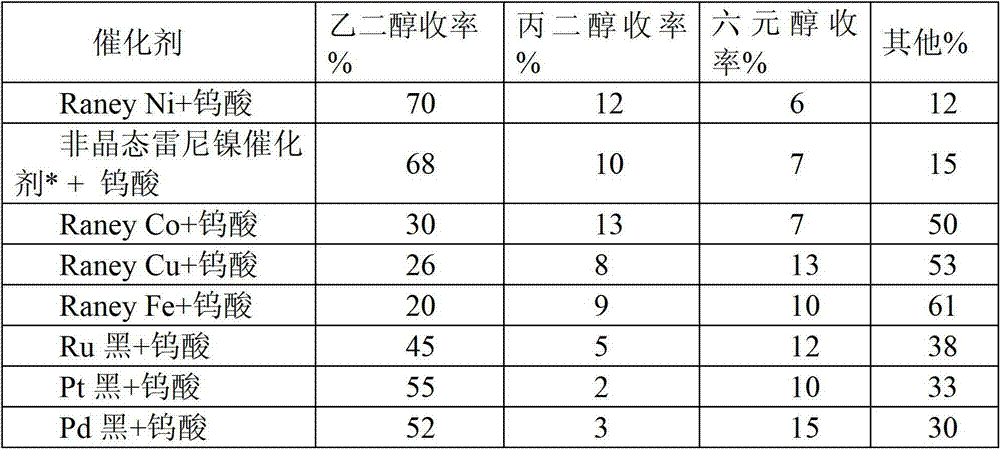
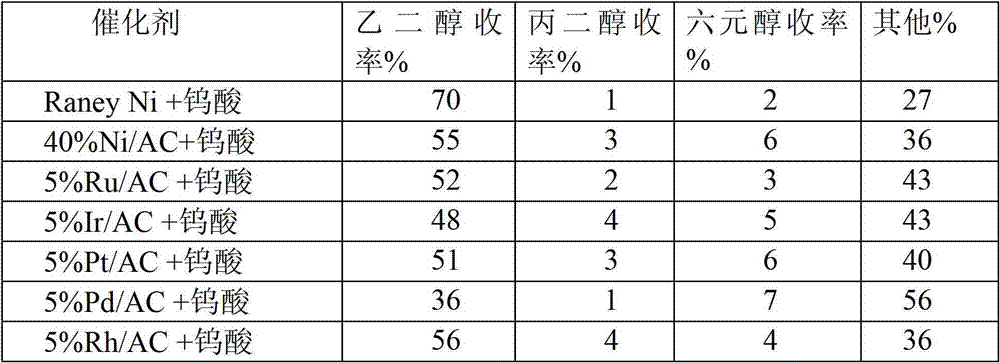
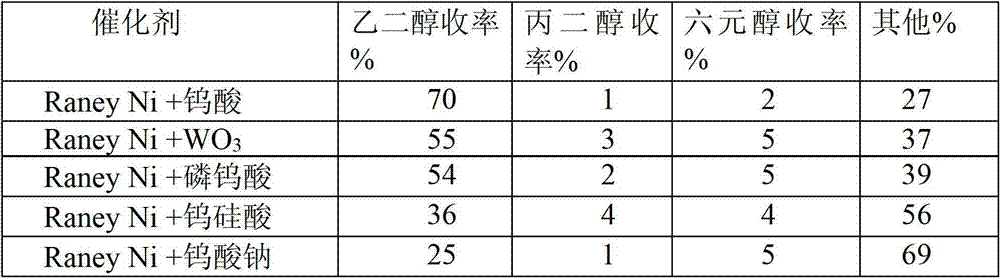
[0026] In the composite catalyst, catalyst A is different metals, catalyst B is tungstic acid, and the reaction conditions are the same as in Example 2. The catalytic conversion results of cellulose on various composite catalysts (catalyst A + catalyst B) (Table 1).
[0027] Table 1 Catalytic conversion results of cellulose on various catalysts
[0028]
[0029] Amorphous Raney nickel catalyst* is ZL-N311 catalyst of Anshan Zhongli Catalyst Factory.
[0030] As shown in Table 1, cellulose can be converted into ethylene glycol with high yield on the combined catalyst involved in the present invention. Among them, the combination catalyst composed of Raney Ni or amorphous nickel alloy catalyst and tungstic acid has the best performance, which is significantly higher than the combination catalyst composed of other non-supported noble metal or non-noble metal catalyst and tungstic acid, and the yield of ethylene glycol It can reach 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com