Medicinal composition for establishing hyperuricemia animal model and application thereof
An animal model and composition technology, which is applied in the directions of active ingredients of heterocyclic compounds, medical preparations containing active ingredients, and pharmaceutical formulations, can solve problems such as large gaps and inconvenient feeding, and achieves short modeling time and modeling. Reasonable method and high modeling success rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
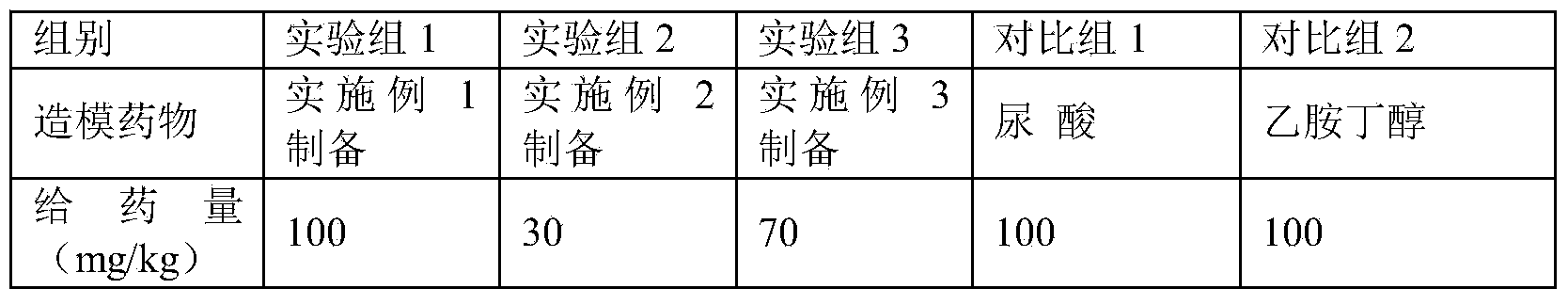
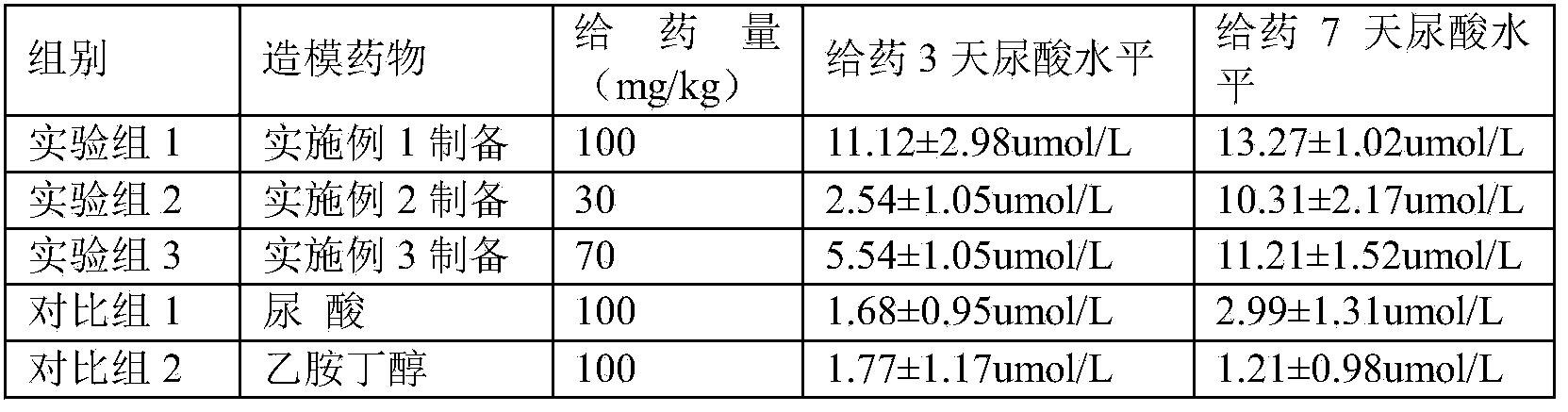
[0025] A pharmaceutical composition for establishing an animal model of hyperuricemia, the pharmaceutical composition consists of 5 parts of ethambutol and 5 parts of uric acid in parts by weight. The modeling time required by using the pharmaceutical composition is short, a stable model can be obtained after 3 days of administration, and the success rate of modeling is high.
Embodiment 2
[0027] A pharmaceutical composition for establishing an animal model of hyperuricemia, the pharmaceutical composition consists of 1 part of ethambutol and 2 parts of uric acid in parts by weight. The required modeling time is short, a stable model can be obtained after 7 days of administration, and the success rate of modeling is high.
Embodiment 3
[0029] A pharmaceutical composition for establishing an animal model of hyperuricemia, the pharmaceutical composition consists of 4 parts by weight of ethambutol and 3 parts of uric acid. The required modeling time is short, a stable model can be obtained after 7 days of administration, and the success rate of modeling is high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com