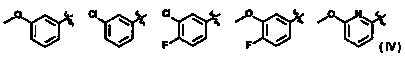
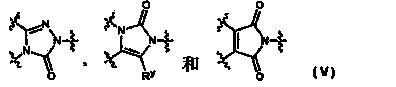
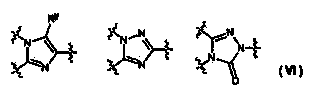
Azole derivative
A technology of azole derivatives and alkyl groups, applied in the field of azole derivatives, can solve the problems such as no reports of compounds with azole skeletons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A-01
[1400] ·Example A-01: 2-[2-(3-chlorophenyl)-4-{4-[2-(piperidin-1-yl)ethyl]phenyl}-1H-imidazol-1-yl Synthesis of ]-N-(propane-2-yl)acetamide
[1401] 【Chemical 226】
[1402]
[1403] CHCl of the compound (533 mg) obtained in Reference Example P-A04 3 (11mL) solution, add Et 3 N (0.28 mL), MsCl (0.12 mL) was added under ice-cold conditions, and stirred at room temperature for 2.5 hours. Under ice-cold conditions, after adding water, use CHCl 3 Extract and concentrate the filtrate under reduced pressure. The obtained residue was subjected to OH silica gel column chromatography (mobile phase: CHCl 3 / EtOAc=70 / 30; v / v) to obtain methanesulfonyl compound (414mg, colorless solid).
[1404] The obtained methanesulfonyl compound (102mg), piperidine (0.042mL), iPr 2 A mixture of NEt (0.073 mL) and MeCN (2.0 mL) was reacted by microwave (100°C, 1.5 hours). Reversed-phase column chromatography (mobile phase: 0.1% TFA MeCN / H 2 O=10 / 90~90 / 10; v / v) refined. Wash the fractions wi...
Embodiment A-02
[1408] ·Example A-02: 2-[2-(3-chlorophenyl)-4-{4-[2-(morpholin-4-yl)ethyl]phenyl}-1H-imidazol-1-yl ]-N-(propan-2-yl)acetamide
[1409] 【Chemical 227】
[1410]
[1411]
[1412]
[1413] .
Embodiment A-03
[1414] ·Example A-03: 2-[2-(3-Chlorophenyl)-4-{4-[2-(2-Oxa-6-azaspiro[3.3]heptane-6-yl)B Base]phenyl}-1H-imidazol-1-yl]-N-(propan-2-yl)acetamide
[1415] 【Chemical 228】
[1416]
[1417]
[1418] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com