Tick cystatin Rhcyst-1, and gene and applications thereof
A cysteine protease and molecular technology, applied to the tick cysteine protease inhibitor molecule and its gene and application fields, can solve the problem that there is no research report on the cysteine protease inhibitor molecule of R. falciparum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
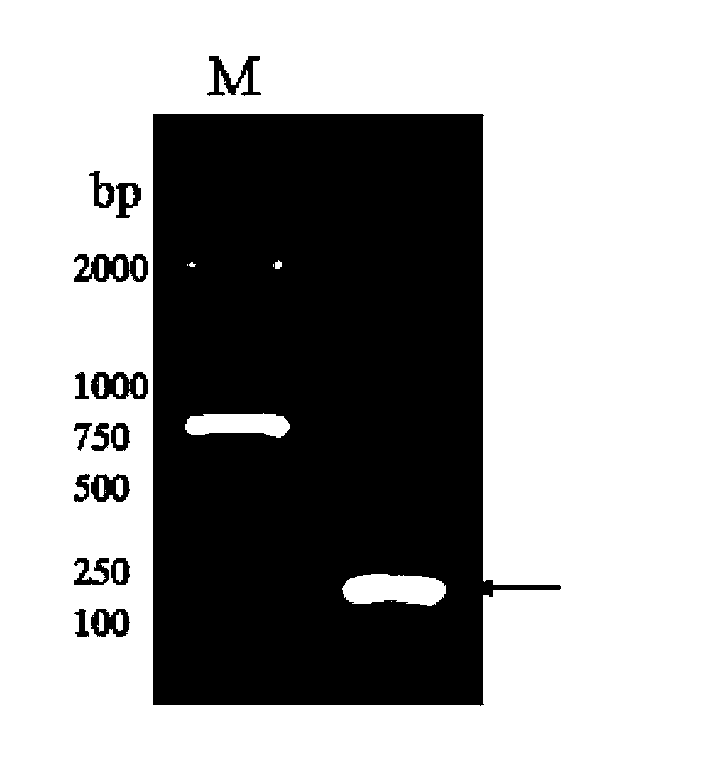
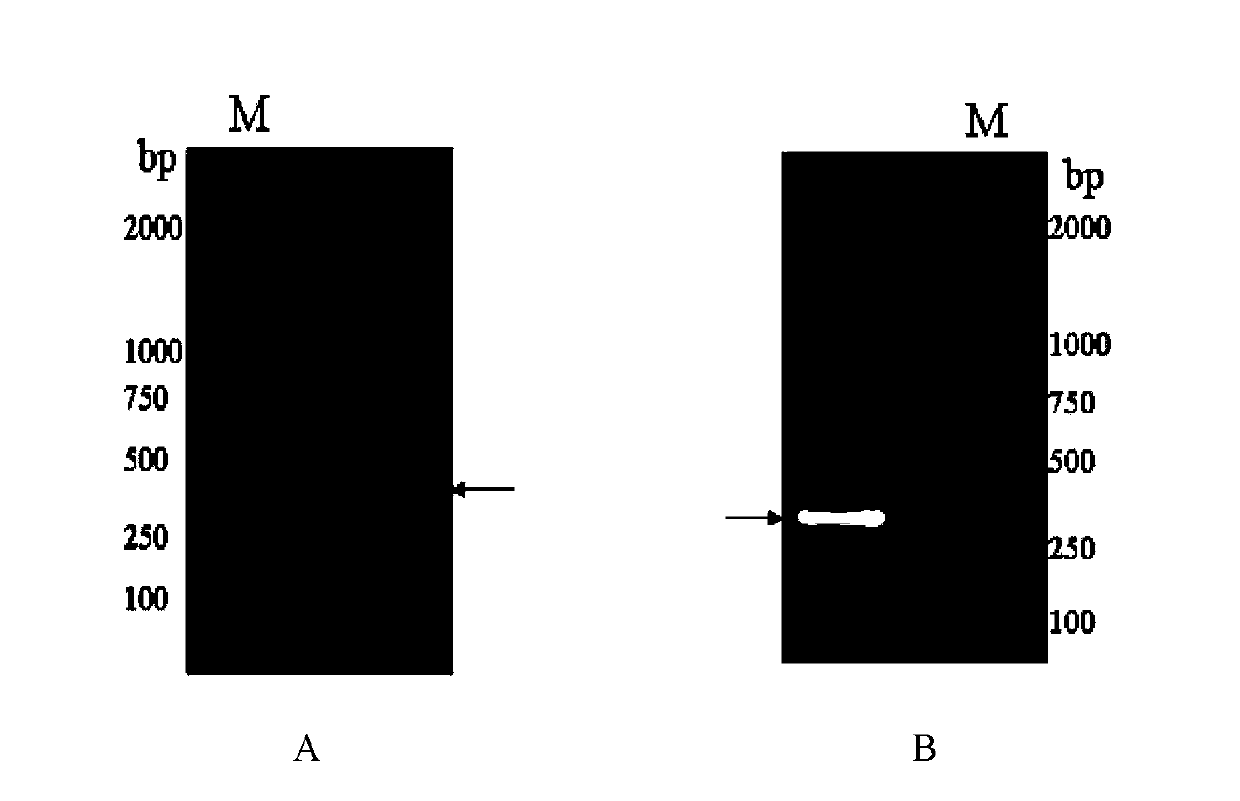
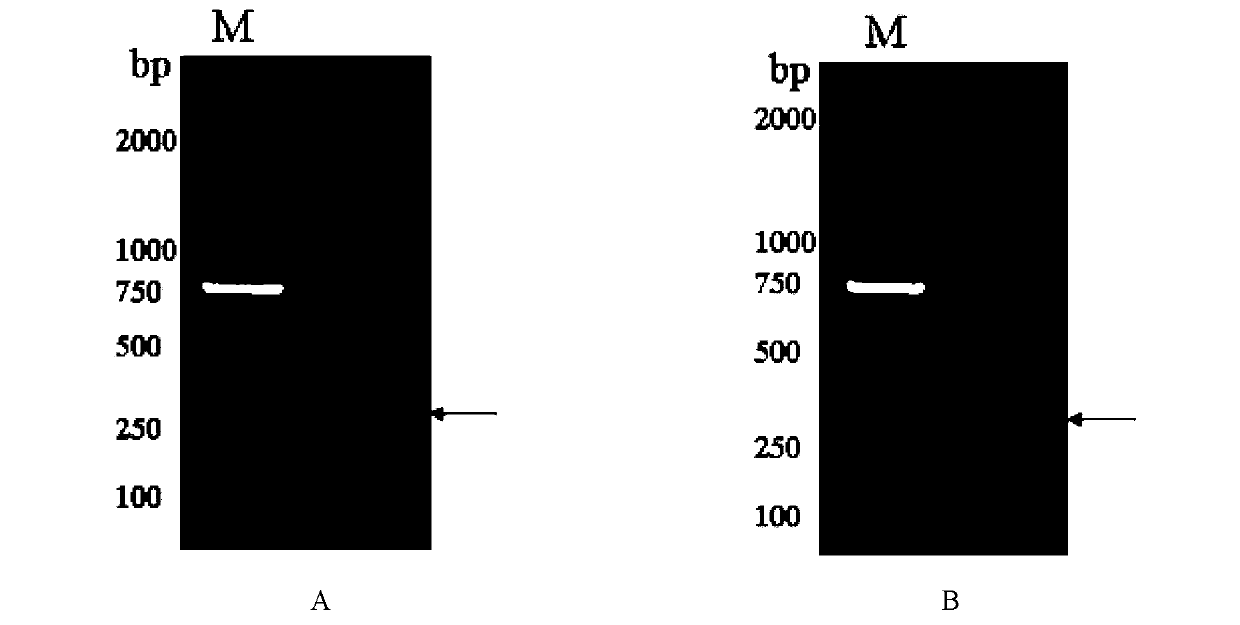
Image
Examples
Embodiment 3
[0124] Example 3 Enzyme Activity Analysis Experiment of Recombinant Protein rRHcyst-1
[0125] 1. Method: To verify the effect of rRHcyst-1 on papain-like cysteine protease by measuring the remaining activity of cathepsin L, B, C, H, S, papain and its corresponding fluorescent substrate after the action of rRHcyst-1 inhibitory activity. The specific method is as follows:
[0126] (1) The cysteine protease reaction solution was prepared according to the formula of 100mM NaAC, 100mM NaCl, 1mM EDTA, 1mg / ml cysteine and 0.005% TritonX-100, and the pH was adjusted to 5.5.
[0127] (2) Six cysteine proteases were prepared to 1.5uM with cysteine protease reaction solution, and their corresponding fluorescent substrates were prepared to 0.5mM.
[0128] (3) The purified RHcyst-1 recombinant protein was formulated into a concentration gradient of 12uM, 6uM, 3uM, 1.5uM, 0.75uM, 0.375uM and 0uM with PBS.
[0129] (4) Add 20ul of RHcyst-1 recombinant protein with seven concent...
Embodiment 4
[0133] Example 4 Anti-Toxoplasma gondii infection experiment of recombinant protein rRHcyst-1
[0134] (1) The experimental animals are Kunming mice, about 25 grams in weight, male. Toxoplasma gondii is the international standard strain RH strain, ascites fluid was collected after infection of mice to collect parasites.
[0135] (2) RHcyst-1 recombinant protein was dissolved in PBS buffer, and the test was divided into high-dose group (200ug / only), medium-dose group (100ug / only), low-dose group (50ug / only), protein control group (GST protein) and a blank control group (PBS), each group of 20 test mice, each mouse was inoculated with 10,000 Toxoplasma gondii intraperitoneally. The recombinant protein was treated by intraperitoneal injection on the 2nd and 4th day after infection with Toxoplasma gondii, and then the mortality and time of death of the mice were observed. The average death time between groups was analyzed by t test to evaluate the effect.
[0136] The mouse mode...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com