Pyrene fluorescence probe and preparation method and application thereof
A fluorescent probe and pyrene-based technology, applied in the field of analytical chemistry, can solve problems such as poor water solubility, poor recognition ability, and fluorescence quenching, and achieve the effects of small environmental impact, strong photostability, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] combined with Figure 1-3 Illustrative Example 1
[0056] Weigh 0.23g of 1-pyrene formaldehyde and 0.11g of 2,3-diaminomalenitrile, and dissolve them in 30mL of absolute ethanol, stir magnetically under reflux for 12h, the solution turns yellow, and the blue fluorescence of 1-pyrene formaldehyde disappears . Anhydrous ethanol was distilled off under reduced pressure to obtain a crude product. The crude product was poured into a saturated sodium chloride solution, extracted and separated by adding dichloromethane, dried and distilled off under reduced pressure, purified by silica gel column chromatography, and eluted with a mixed solution of petroleum ether / ethyl acetate. The eluent was evaporated and dried to obtain a yellow solid, which was 2-amino-3-(1-pyrenylmethyleneamino)maleonitrile, abbreviated as PYMN.
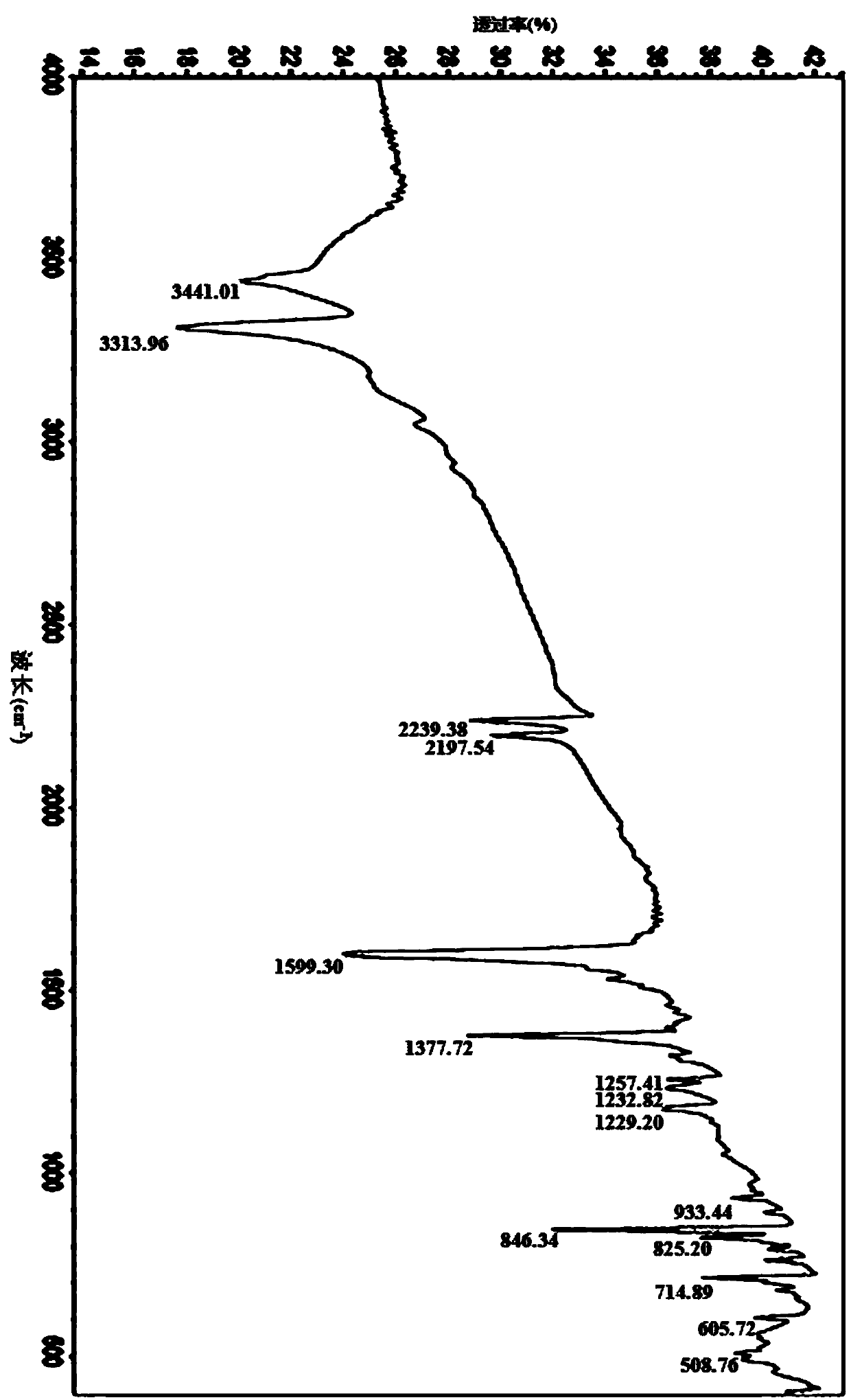
[0057] Carry out nuclear magnetic resonance analysis and infrared analysis to the PYMN that embodiment 1 obtains; figure 1 for PYMN in DMSO-d 6 middle 1 H...
Embodiment 2
[0059] combine Figure 4-7 Illustrative Example 2
[0060] (1) Using pure water and H 2 o 2 , NaAcO, Na 2 C 2 o 4 、Na 2 CO 3 、NaNO 2 、NaNO 3 、Na 2 S, Na 2 SO 3 、Na 2 S 2 o 3 , NaCl, NaClO, NaClO 3 , NaBr, KBrO 3 , KI and KIO 3 , were prepared in a volume of 100mL, and contained a concentration of 1.0×10 -2 mol / L of H 2 o 2 、AcO - 、C 2 o 4 2- , CO 3 2- , NO 2 - , NO 3 - , S 2- , SO 3 2- , S 2 o 3 2- , Cl - , ClO - , ClO 3 - , Br-, BrO 3 - , I - , IO 3 - aqueous solution;
[0061] (2) Dissolve PYMN in absolute ethanol, and make the PYMN concentration 1.0×10 -5 mol / L probe solution;
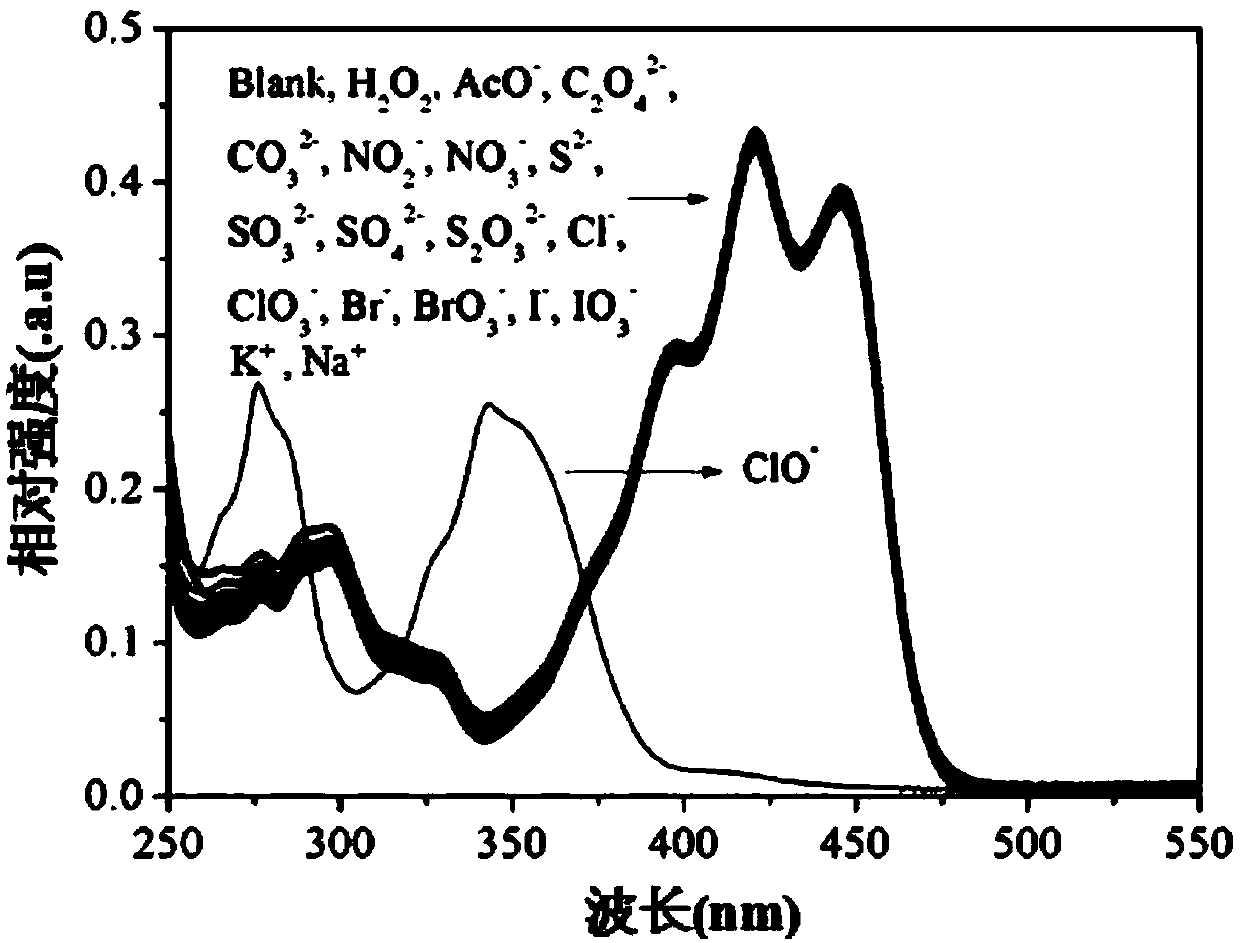
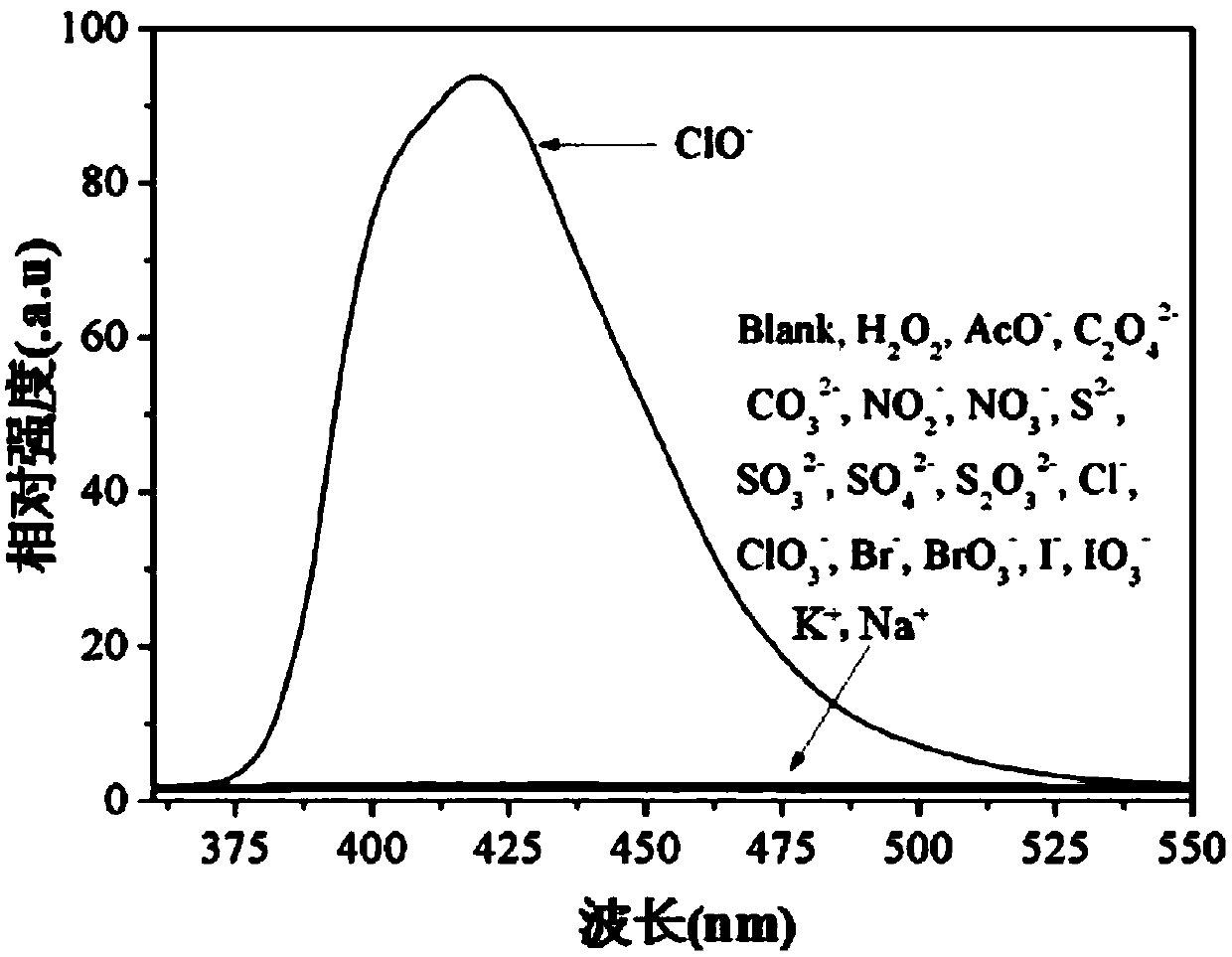
[0062] (3) Take 17 parts of the probe solution with a volume of 3 mL, and add 30 μL of the aqueous solution containing different ions in step (1) and 30 μL of pure water to it respectively to obtain a mixed solution;
[0063] (4) At room temperature, observe the color change of the mixed solution and the color change under 365nm ultraviolet light irrad...
Embodiment 3
[0067] combine Figure 6-8 Illustrative Example 3
[0068] (1) Using pure water and CaCl 2 , MgCl 2 , FeCl 2 , FeCl 3 , ZnCl 2 , CuCl 2 , were prepared in a volume of 100mL, and contained a concentration of 1.0×10 -2 mol / L Ca 2+ , Mg 2+ , Fe 2+ , Fe 3+ , Zn 2+ 、Cu 2+ aqueous solution;
[0069] (2) Dissolve PYMN in absolute ethanol, and make the PYMN concentration 1.0×10 -5 mol / L probe solution;
[0070] (3) Take 6 parts of the probe solution with a volume of 3 mL, and add the aqueous solution containing different ions with a volume of 30 μL to it respectively to obtain a mixed solution;
[0071] (4) At room temperature, observe the color change of the mixed solution and the color change under 365nm ultraviolet light irradiation, and detect its absorption spectrum and fluorescence spectrum respectively.
[0072] From Figure 8 It can be seen that the probe solution containing Ca 2+ , Mg 2+ , Fe 2+ , Fe 3+ , Zn 2+ or Cu 2+ There is no characteristic fluore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com