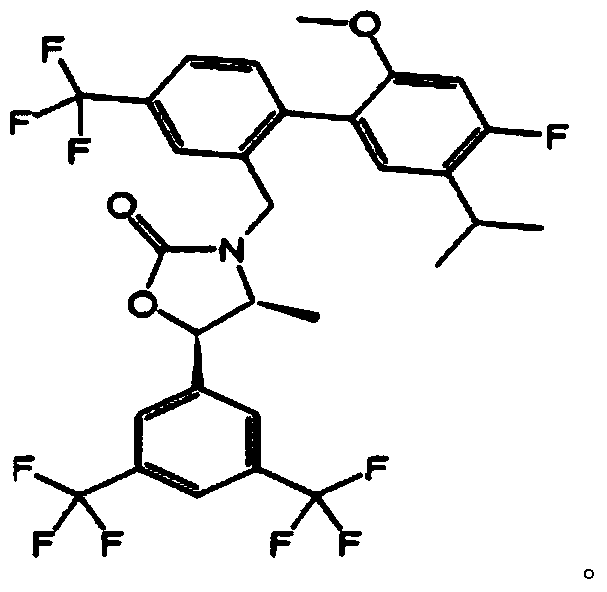
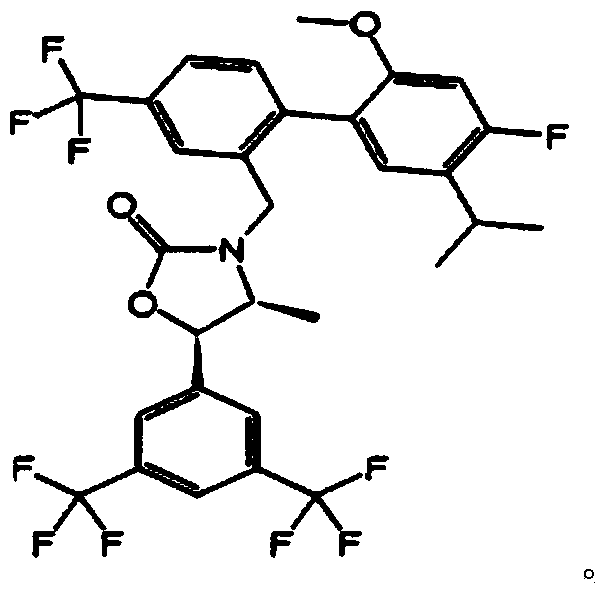
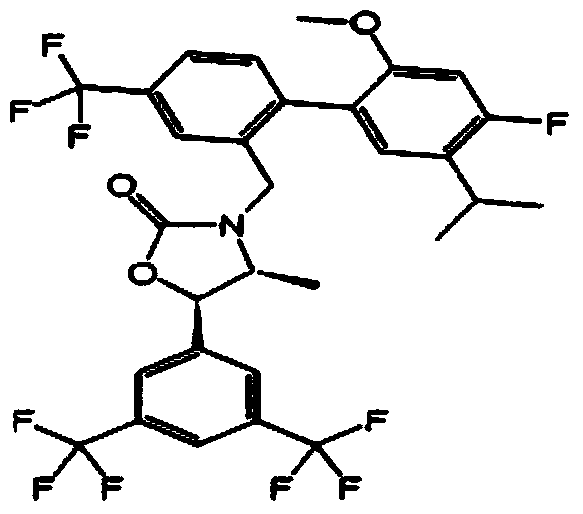
Pharmaceutical composition for preventing or treating hypertriglyceridemia or hypertriglyceridemia-associated diseases
A technology for hypertriglyceridemia and a composition is applied in the field of pharmaceutical compositions for preventing or treating hypertriglyceridemia or hypertriglyceridemia-related diseases, and can solve the problem of increasing atherosclerosis. risks, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Evaluation of triglyceride inhibitory activity in hypercholesterolemia-induced animals
[0032] (1) Test method
[0033] Male New Zealand white rabbits were used as test animals. All animals except the G1 group animals (negative control group, n=4) were fed with a high-cholesterol meal sterilized by irradiation, i.e. DYET #620007 (Purina #5321 food, containing 1% cholesterol, Dyets, Inc., Bethlehem ( Bethlehem), PA18017), which was purchased from Central Lab. Animal Inc. To induce hypercholesterolemia, animals were provided with meals over 8 weeks. After blood samples are collected from animals, they are subjected to serum biochemical analysis. Animals with a total cholesterol level of approximately 870 mg / dL were selected as hypercholesterolemia induced animals. The test material was dissolved in saline containing 0.5% sodium carboxymethylcellulose and 1% Tween80, and then administered directly to the stomach using an oral syringe fitted with a latex cath...
Embodiment 2
[0042] Example 2 Evaluation of Triglyceride Inhibitory Activity in Animals Inducing Hypertriglyceridemia and Hypercholesterolemia
[0043] (1) Test method
[0044] Male New Zealand white rabbits were used as test animals. All animals except the G1 group animals (negative control group) were fed an irradiated hypertriglyceridemic and high cholesterol meal, DYET #621082 (Purina #5321 food with 0.5% cholesterol, 14% coconut oil and 2% maltodextrin, Dyets Company, Bethlehem, PA 18017), which were purchased from Saeronbio Company. To induce hypertriglyceridemia and hypercholesterolemia, animals were provided with meals over 4 weeks. After blood samples are collected from animals, they are subjected to serum biochemical analysis. Animals showing significant changes in total cholesterol levels and triglyceride levels compared to untreated controls were selected. Selected animals were divided into 4 groups according to total cholesterol levels and triglyceride levels, whereby all ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com