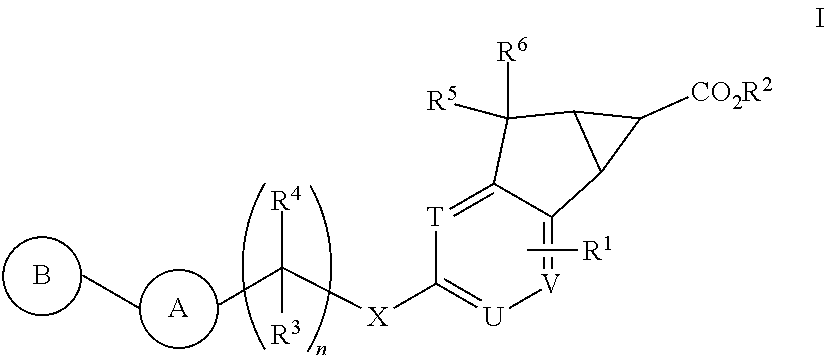
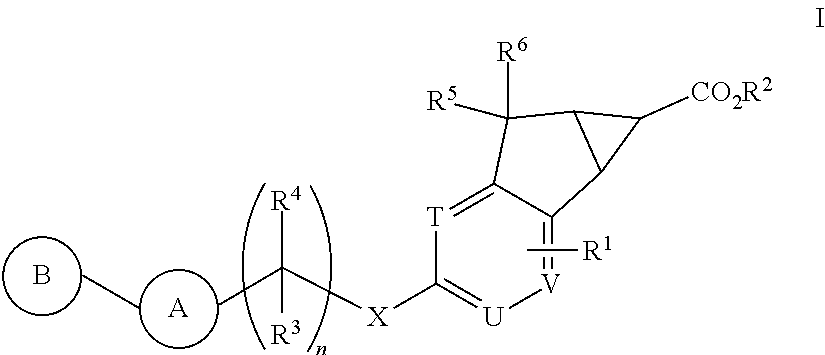
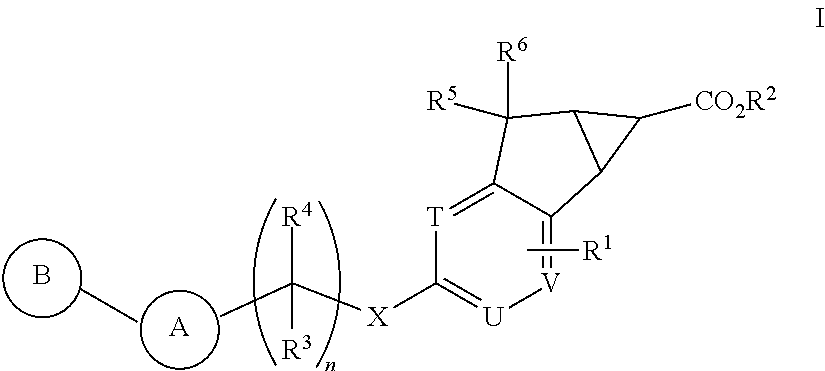
Antidiabetic tricyclic compounds
a tricyclic compound and antidiabetic technology, applied in the field of antidiabetic tricyclic compounds, can solve the problems of increased and premature morbidity and mortality, inadequate insulin-mediated repression of lipolysis in adipose tissue, oxidation and storage of glucose in muscle,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1-9
4-Hydroxy-1,1a,6,6a-tetrahydro-3-aza-cyclopropa[a]indene-1-carboxylic acid ethyl ester (1-9)
[0537]
Step A: (4-Bromo-pyridin-2-yl)-bis-(4-methoxy-benzyl)-amine (1-2)
[0538]
[0539]To a suspension of sodium hydride (60% in oil, 93 g, 2.32 mol) in DMF (1.8 L), was added compound 1-1 (100 g, 0.58 mol) in DMF (500 mL) slowly at 0° C. Then the resulting mixture was allowed to stir at r.t. for 0.5 h under N2 protection. PMBCl (227 g, 1.45 mol) was added to the above mixture and the temperature was kept between 0-10° C. After addition, the mixture was allowed to stir at room temperature for 2 h. The mixture was carefully cpoured into ice water, and the resulting solid precipitate was collected, filtered and washed with PE (150 mL×3). The filtrate was concentrated to afford compound 1-2. MS (ESI) m / e (M+H+): 414.1 / 416.1.
Step B: (4-Bromo-5-iodo-pyridin-2-yl)-bis-(4-methoxy-benzyl)-amine (1-3)
[0540]
[0541]To a stirred solution of compound 1-2 (140 g, 0.34 mol) in DMF (2.8 L), was added NIS (115 g, ...
reference example 2-4
4-[2-Fluoro-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzyloxy]-1,1a,6,6a-tetrahydro-3-aza-cyclopropa[a]indene-1-carboxylic acid ethyl ester (2-4)
[0574]
Step A: [2-Fluoro-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-methanol (2-2)
[0575]
[0576]To a stirred solution of compound 2-1 (25 g, 120 mmol) in dioxane (400 mL), was added bis(pinacolato)diboron (45 g, 180 mmol), Pd(dppf)Cl2 (4.4 g, 6.0 mmol) and KOAc (23.5 g, 240 mmol). The resulting mixture was heated to 110° C. under N2 overnight. The mixture was then concentrated to afford the crude product, which was purified by column chromatography on silica gel (PE:EA=20:1) to give compound 2-2. MS (ESI) m / z: 253 (M+H+).
Step B: 2-(3-Bromomethyl-4-fluoro-phenyl)-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane (2-3)
[0577]
[0578]To a stirred solution of compound 2-2 (7 g, 28 mmol) in THF (80 mL), was added PBr3 (7.6 g, 28 mmol) dropwise at 0° C. The resulting mixture was stirred at 0° C. for 1 h. H2O (50 mL) was added to the mixtu...
reference example 2-5
[0582]1-NMR (400 MHz, CDCl3) δ: 8.10 (s, 1H), 7.88 (s, 1H), 7.75 (d, 1H, J=4.0 Hz), 753(d, 1H, J=4.0 Hz), 7.37 (t, 1H, J=8.0 Hz), 6.61 (s, 1H), 5.36 (s, 2H), 4.12 (dd, 2H, J=2.0 and 7.2 Hz), 3.23 (dd, 1H, J=6.0 and 12.0 Hz), 2.98 (d, 1H, J=8.8 Hz), 2.88 (d, 1H, J=2.8 Hz), 2.43-2.39 (m, 1H), 1.34 (s, 12H), 1.26-1.23 (m, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com