Antibodies specific for Trop-2 and their uses
A trop-2, specific technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc., can solve difficult problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0364] Example 1: Detection of Antibody Binding Affinity of Recombinant Anti-Trop-2 Mouse Antibody
[0365] The affinity of the anti-Trop-2 mouse antibody produced by the hybridoma was measured by surface plasmon resonance using a research-grade CM5 sensor chip (Biacore TM AB, Uppsala, Sweden - now GE Healthcare) Biacore TM 2000 or 3000 biosensors. Anti-mouse IgG is the first amine coupled to the surface of the CM5 chip. Multiple anti-Trop-2 mouse antibodies were then captured by anti-mouse IgG. Monomeric Trop-2 ectodomain was prepared by papain digestion of Trop-2-Fc fusion protein, followed by 3-fold serial dilution as the analyte for injection. The affinity of the anti-Trop-2 mouse antibody ranges from 7.5 to 31.8nM. See Table 4.
[0366] Table 4
[0367]
Embodiment 2
[0368] Example 2: Domain mapping of recombinant anti-Trop-2 mouse antibody
[0369] Domain mapping was performed by exchanging the Trop-2 ectodomain with the equivalent region of Trop-1 (EpCAM) or mouse Trop-2. See Figure 4 and 5 . Anti-Trop-2 antibodies 3E9, 6G11, 7E6 and 18B1 (expressed as recombinant mouse IgG2a) do not bind to human Trop-1 or mouse Trop-2, whereas 15E2 binds to mouse Trop-2 but not to human Trop-2 1 combined. These hybrid proteins were expressed as human Fc fusion proteins in 293F cells. Binding of anti-Trop-2 antibodies to these domain hybrids was determined by Biacore. Anti-Trop-2 antibodies are defined to recognize certain human Trop-2 domains when domain swapping results in loss or reduction of binding. Anti-Trop-2 antibodies 3E9, 7E6 and 15E2 bind domains 3 and 4, whereas antibodies 6G11 and 18B1 bind domain 1. See Table 5. Definitions of the different domains of Trop-2 can be found, for example, in Chong et al., J. Biol. Chem. 276(8):5804-13...
Embodiment 3
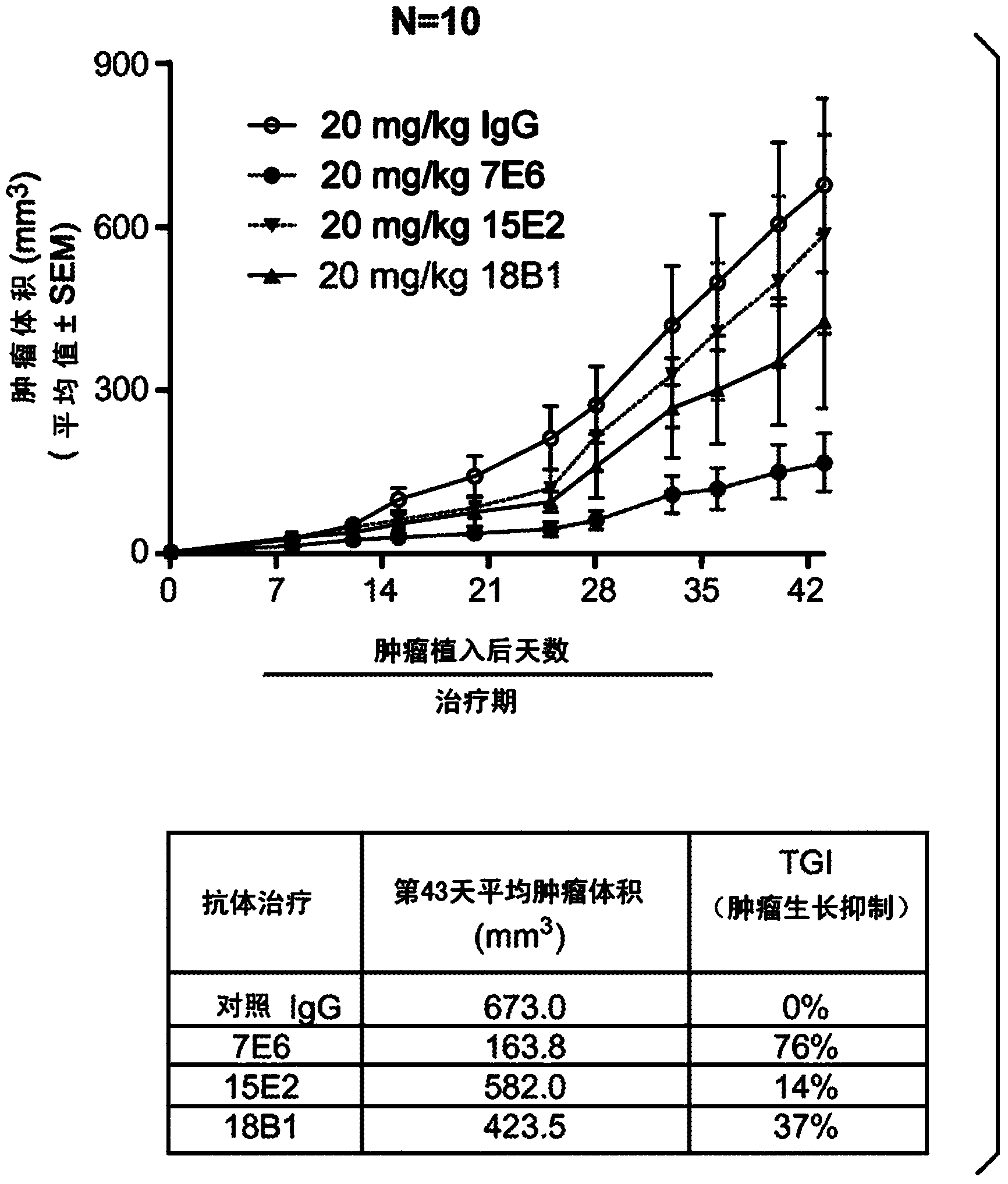
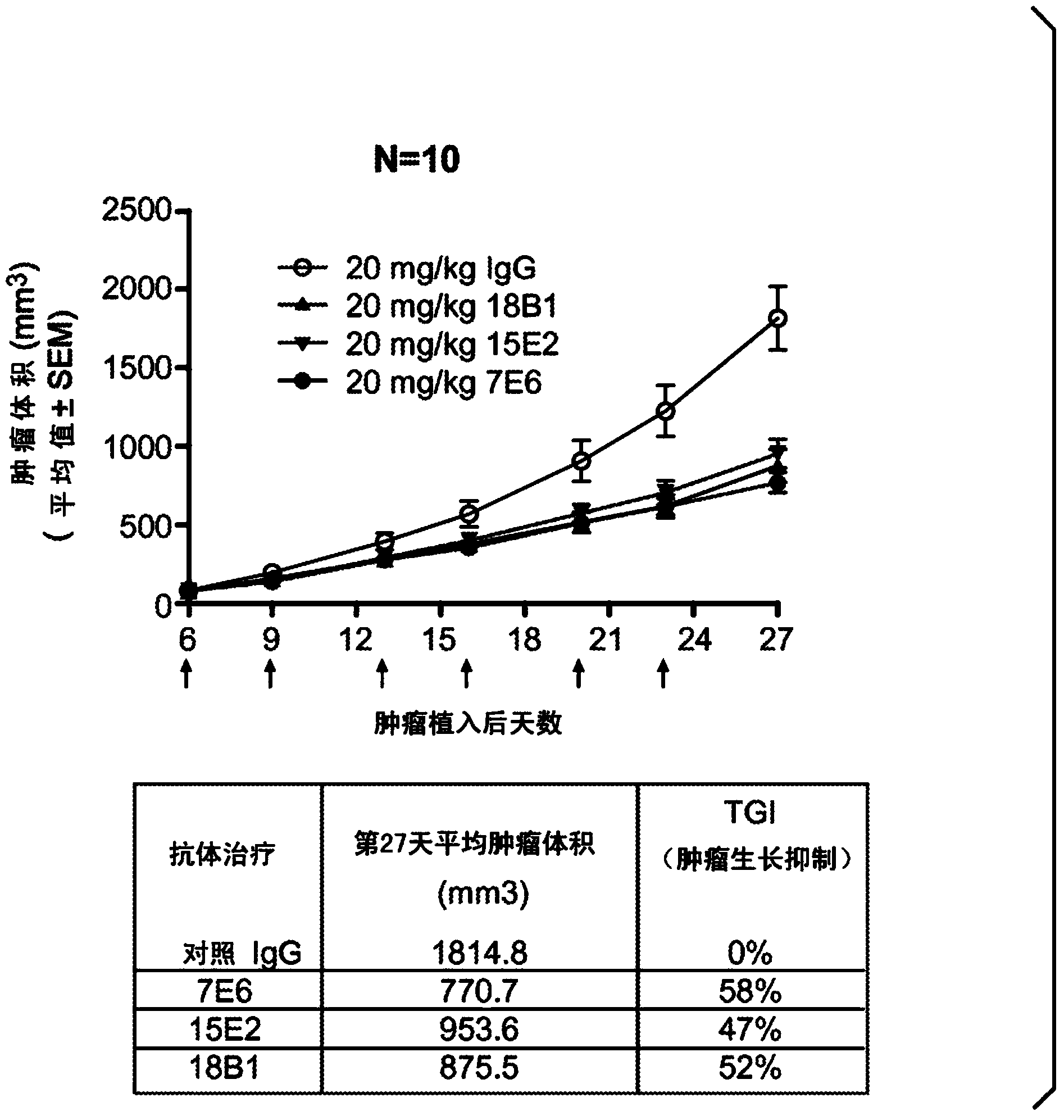
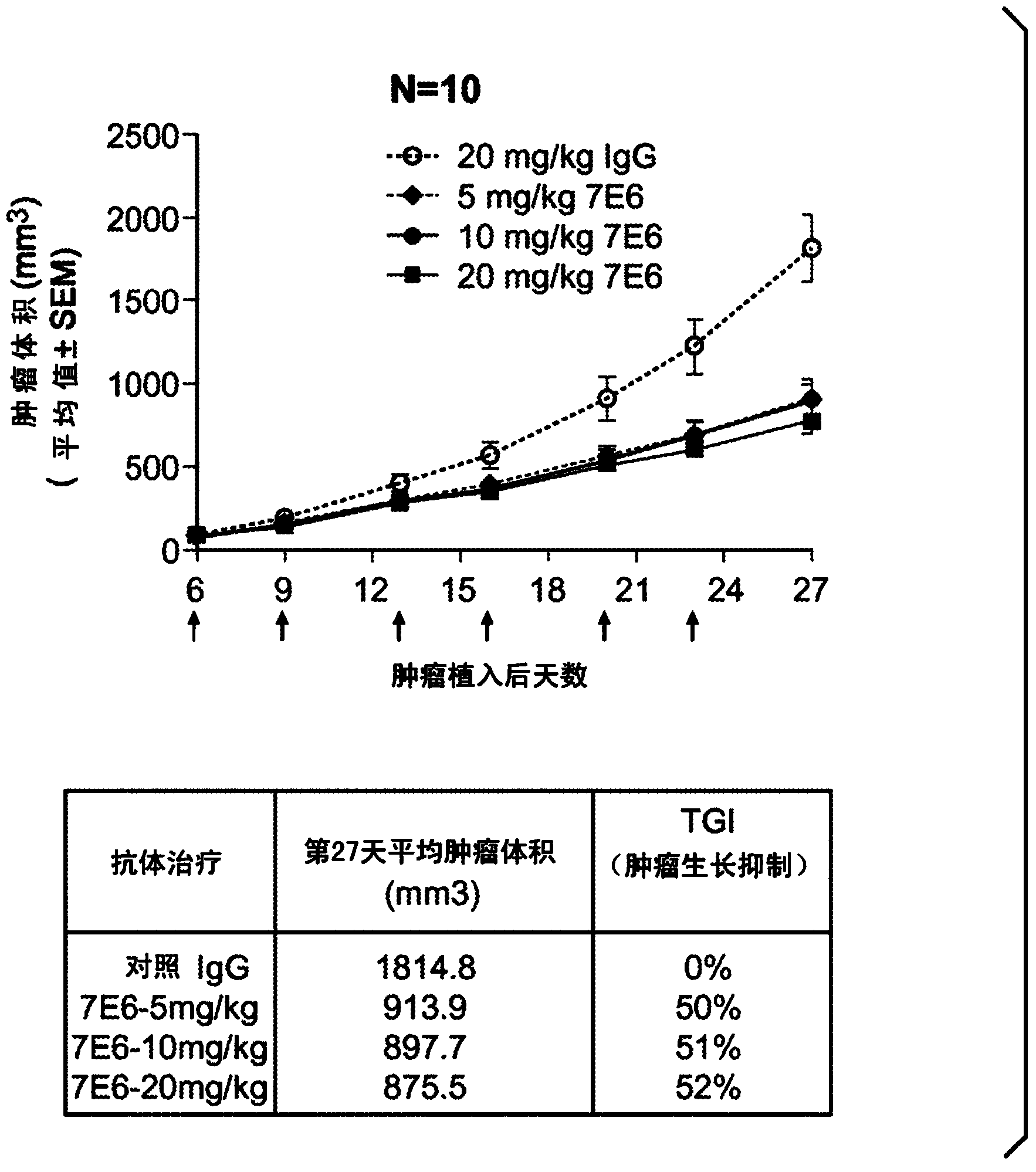
[0375] Example 3: In vivo curative effect study of Colo205 xenograft model
[0376] In vivo efficacy studies of anti-Trop-2 mouse IgG were performed in a target-expressing colo205 xenograft model. One million colo205 colon cancer cell lines were implanted subcutaneously into 5- to 8-week-old nu / nu mice (day 0). On the next day (Day 1), the mice were randomly assigned to different treatment groups (control IgG, 7E6, 15E2 or 18B1 groups; n=10 / group) according to their body weight. 20 mg / kg of anti-Trop-2 antibody and control mIgG in each treatment group were administered by rapid injection through the tail vein, three times a week for a total of 12 doses. Body weight changes of all experimental animals were monitored daily. Tumor volume was measured twice a week by calipers and calculated using the following formula: tumor volume = (length x width 2 ) / 2. Study when the tumor volume reaches 2000mm 3 terminated before. TGI (percent tumor growth inhibition) was determined by ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com