Plasma endotoxin detection kit quality control product and preparation method thereof
A technology for detection kits and quality control products, which is applied in the field of plasma endotoxin detection kit quality control products and its preparation, can solve the problems of large differences in plasma, achieve long validity period, reduce matrix effect, and facilitate storage and transportation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
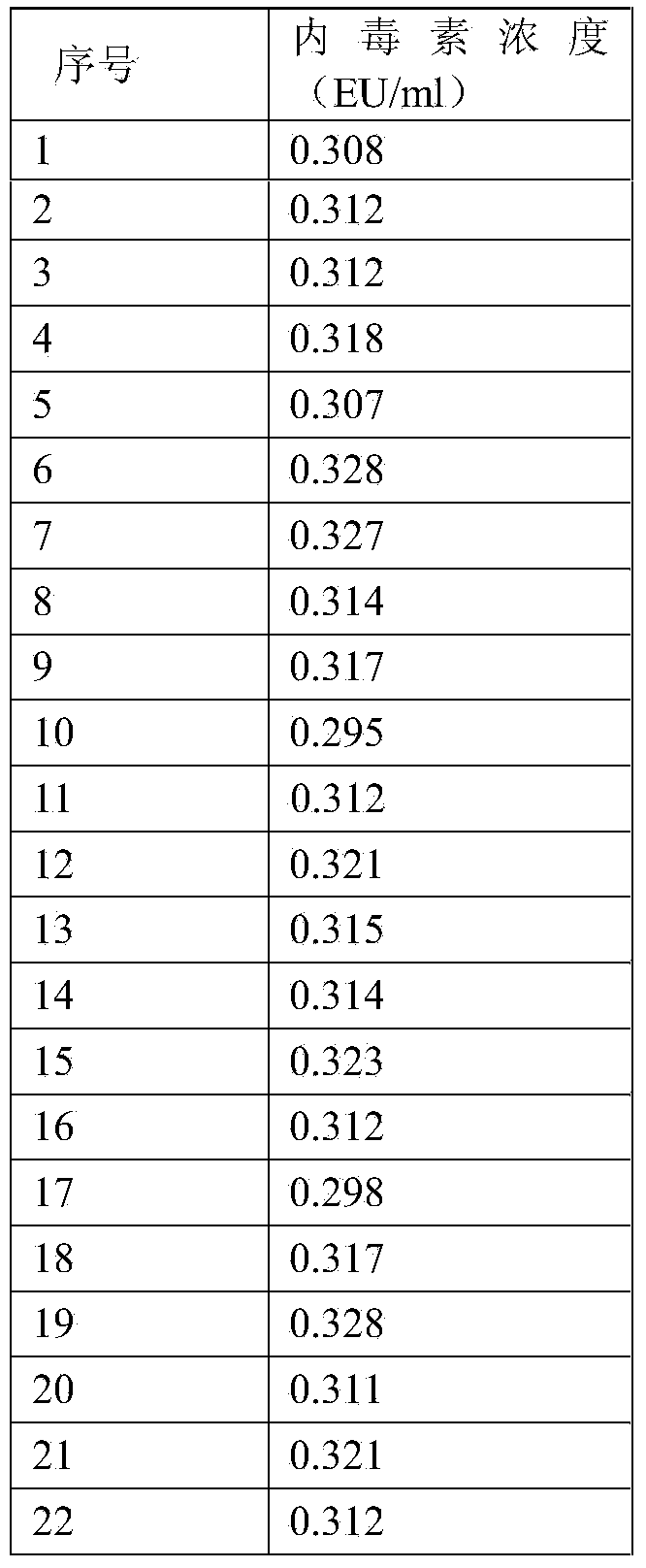
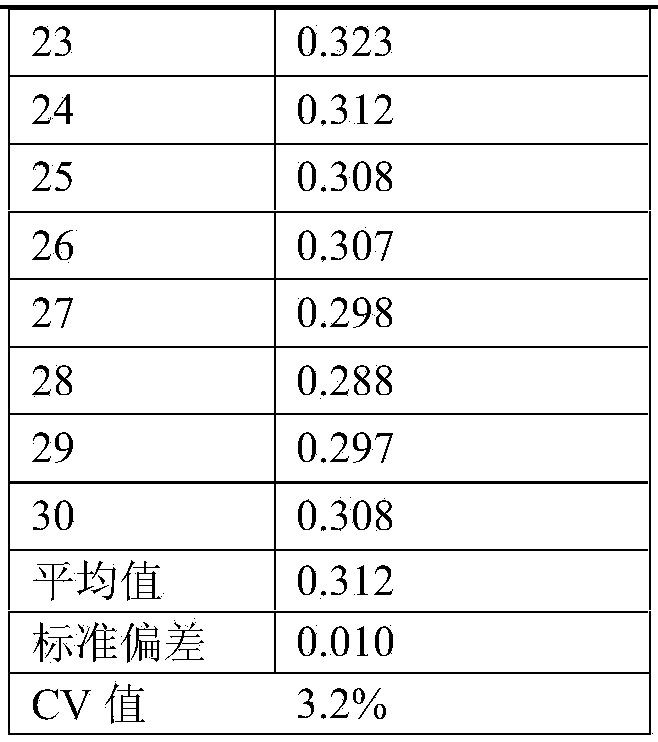
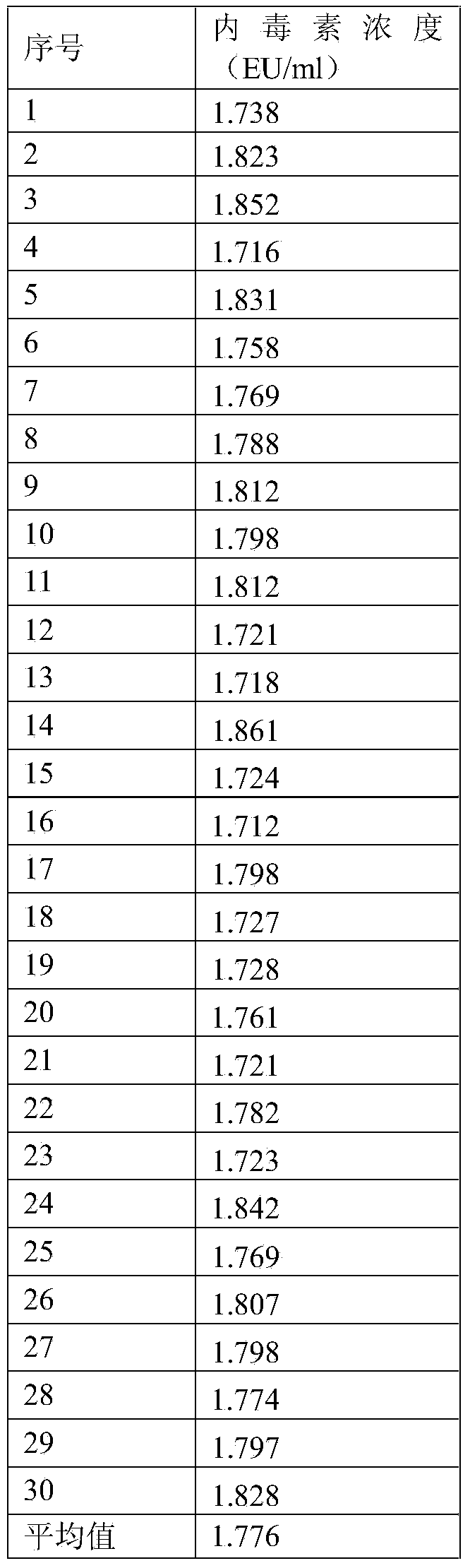
[0021] A plasma endotoxin detection kit quality control product, including a high-value quality control product and a low-value quality control product; the components of the high-value quality control product are finished human plasma with an endotoxin content of 1.8-2.2 EU / ml; The components of the low-value quality control product are finished human plasma with an endotoxin content of 0.3-0.7 EU / ml.
[0022] As a preferred embodiment of the embodiment, the matrix used in the high-value quality control and the low-value quality control is finished human plasma with an endotoxin content less than 0.01 EU / ml.
[0023] As a preferred mode of the embodiment, the high-value quality control substance and the low-value quality control substance are in the form of dry powder after freeze-drying.
[0024] As a preferred mode of the embodiment, the sample processing solution added to the endotoxin detection kit or the bacterial endotoxin test water is reconstituted into a liquid durin...
Embodiment 2
[0026] A method for preparing a plasma endotoxin detection kit quality control product, comprising the following steps:
[0027] Step 1. Use 200ml of finished human plasma, and perform quantitative detection of endotoxin according to the requirements of the plasma endotoxin detection kit instructions. If the endotoxin content is less than 0.01EU / ml, it can be used as a quality control matrix;
[0028] Step 2: Prepare 2 tubes of 100EU endotoxin standard solution, take endotoxin national working standard or endotoxin working standard, add 1ml bacterial endotoxin test water according to the instructions, vortex and mix for more than 15 minutes, and get 100EU / ml endotoxin Toxin standard solution;
[0029] Step 3: Take the endotoxin standard solution prepared in step 2, add 0.3ml of endotoxin standard solution to the quality control matrix in step 1 according to the ratio of adding 0.3ml of endotoxin standard solution per 100ml of quality control substance matrix, and use a magneti...
Embodiment 3
[0042] A method for preparing a plasma endotoxin detection kit quality control product, comprising the following steps:
[0043] Step 1. Use 200ml of finished human plasma, and perform quantitative detection of endotoxin according to the requirements of the plasma endotoxin detection kit instructions. If the endotoxin content is less than 0.01EU / ml, it can be used as a quality control matrix;
[0044] Step 2: Prepare 2 tubes of 100EU endotoxin standard solution, take endotoxin national working standard or endotoxin working standard, add 1ml bacterial endotoxin test water according to the instructions, vortex and mix for more than 15 minutes, and get 100EU / ml endotoxin Toxin standard solution;
[0045] Step 3: Take the endotoxin standard solution prepared in step 2, add 0.5ml of endotoxin standard solution to the quality control matrix in step 1 according to the ratio of adding 0.5ml of endotoxin standard solution per 100ml of quality control substance matrix, and use a magneti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com