Arginine aspirin powder injection split filling process and automatic split filing system thereof
A technology for arginine aspirin and injection, which is applied to the subpackaging process of arginine aspirin powder for injection and the field of its automatic subpackaging system, can solve the problems of unstable drug properties, easy deterioration of drugs, and the existence of bacteria. , to achieve good medicinal effect, good drug quality and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
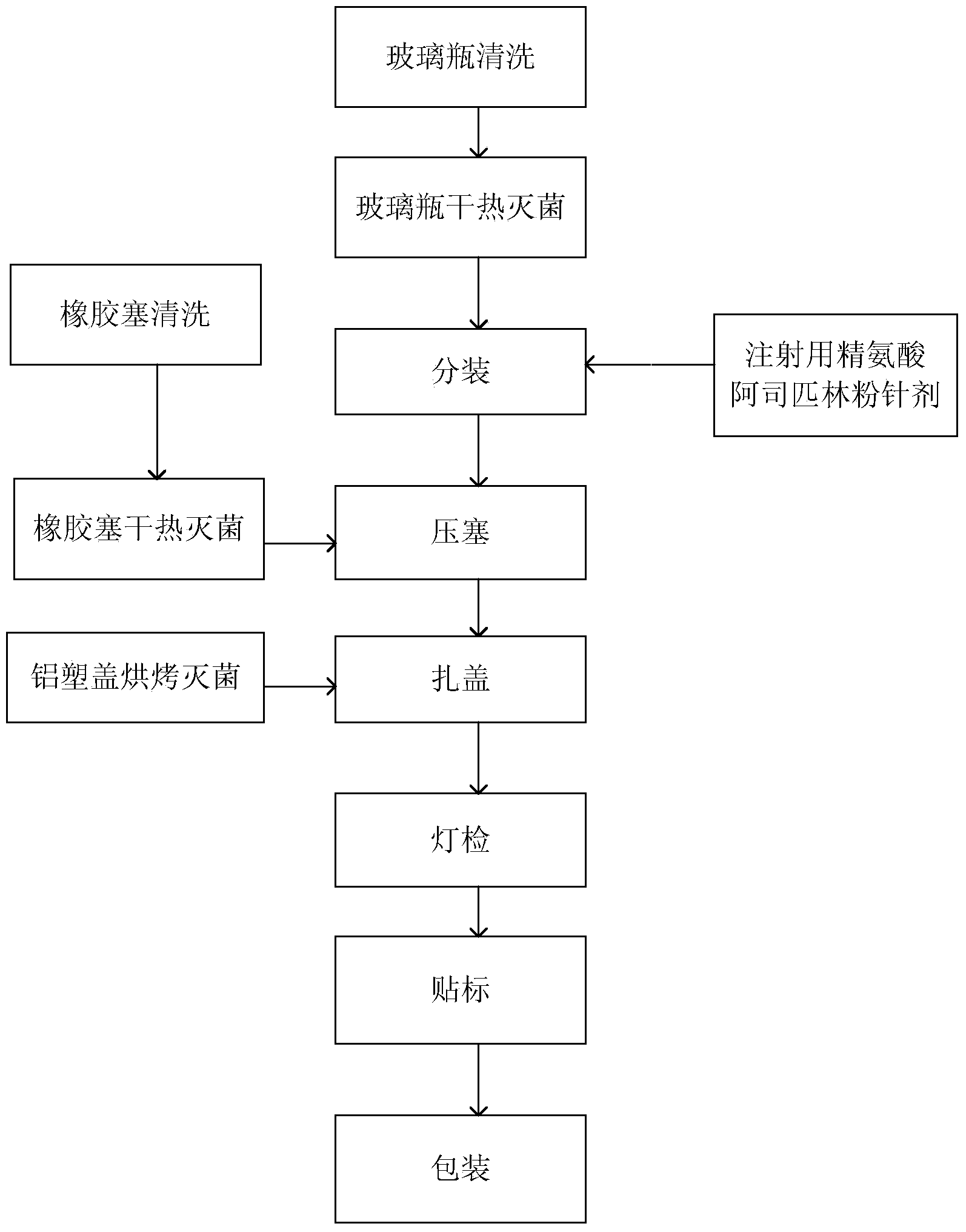
[0014] Now in conjunction with specific examples, the packing process of arginine aspirin powder for injection of the present invention is further described, specifically as follows:
[0015] refer to figure 1 , the sub-packaging process of arginine aspirin powder for injection of the present invention comprises the following steps: 1, cleaning the glass bottle, placing the 10ml glass bottle in a cleaning machine for cleaning and blowing dry with compressed air; 2, extinguishing the glass bottle sterilize, put the cleaned glass bottle in the first dry heat sterilization device, sterilize at 320°C for 6 minutes, and then cool it for later use; 3. Clean the rubber stopper, put the rubber stopper in the rubber stopper washing machine for cleaning; 4. Sterilize the rubber stopper, put the cleaned rubber stopper in the second dry heat sterilization device, sterilize it at 121°C for 120 minutes, and then cool it for later use; 5. Sterilize the aluminum-plastic cover, put the aluminu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com