Method for controlling residual lithium on surface of layered nickelic positive electrode material
A cathode material and control layer technology, applied in the field of modification of layered high-nickel cathode materials, can solve the problems of destroying the material structure and electrochemical performance, and achieve the effect of good electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
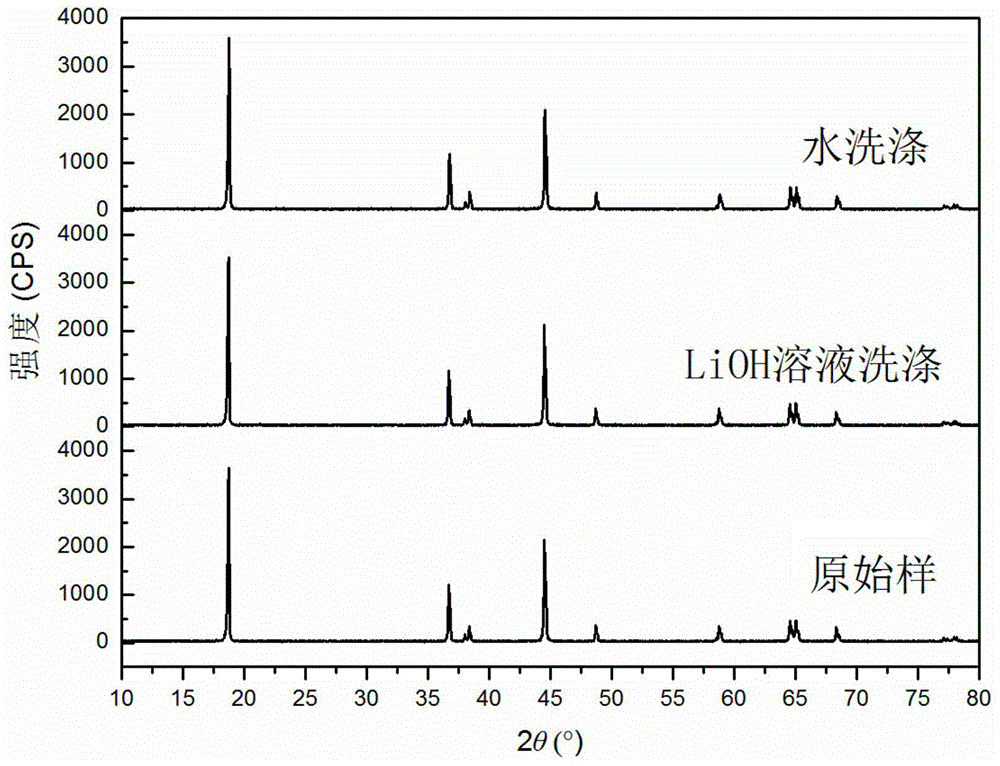
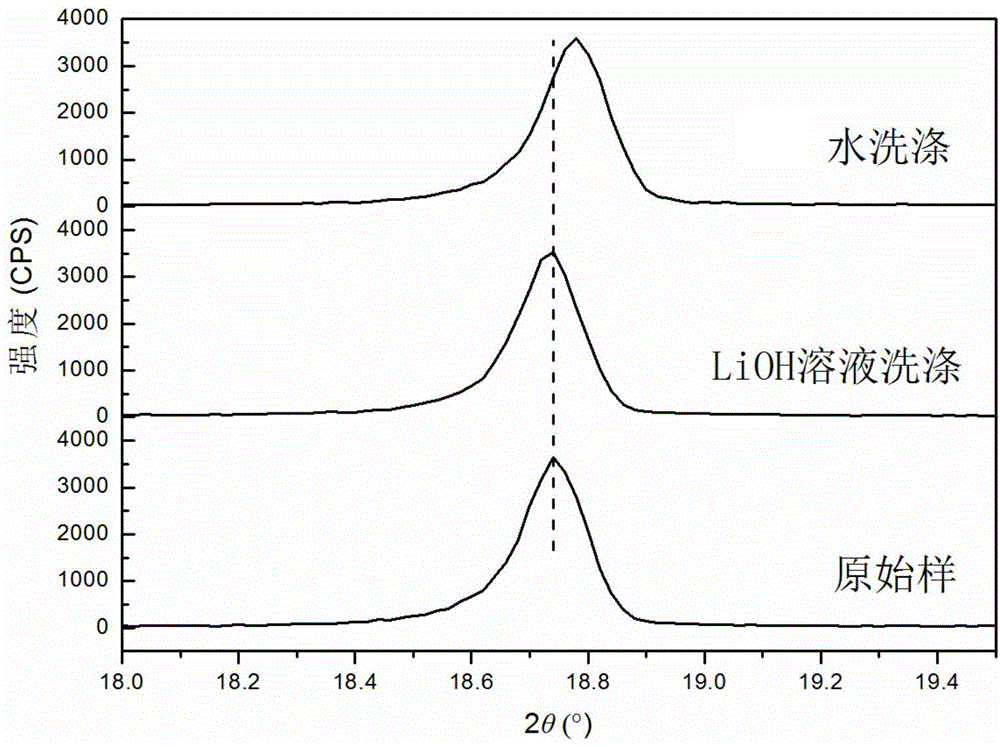
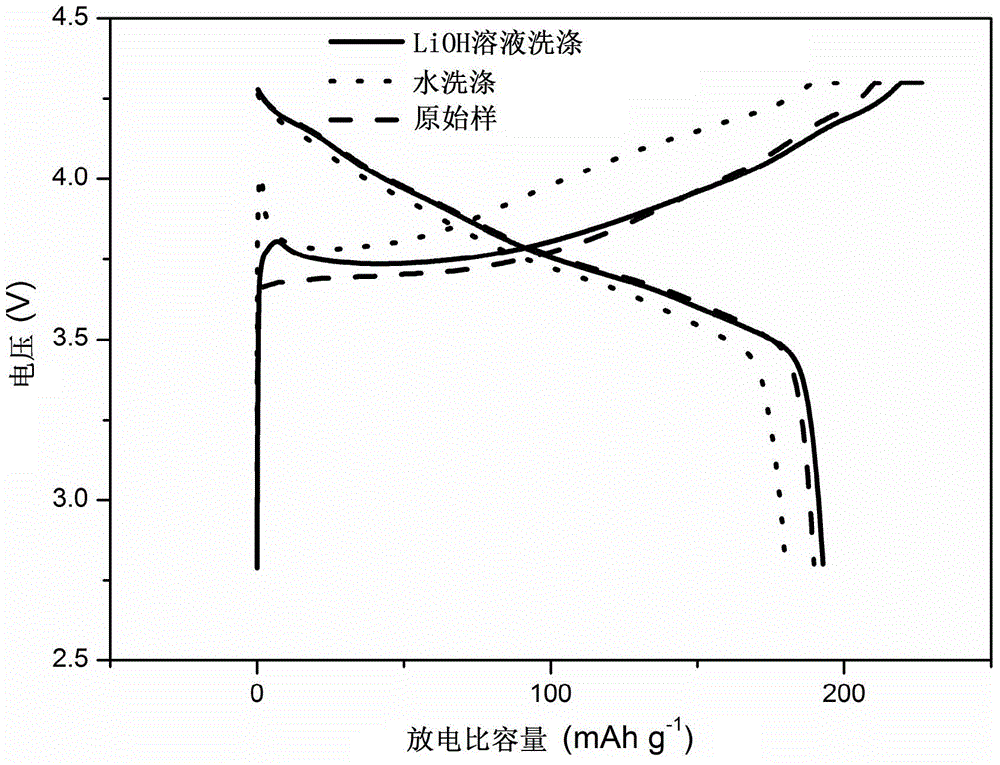
[0030]With Ni 0.8 co 0.15 Al 0.05 (OH) 2 As the precursor, with LiOH·H 2 O is the lithium source (slightly excessive), and the high-nickel cathode material LiNi is prepared by a high-temperature solid-phase method. 0.8 co 0.15 Al 0.05 o 2 , to detect the amount of residual lithium in the material to be washed, to be washed. Configure LiOH washing solution: obtain the solubility s (g / 100g water) of LiOH in pure water at the current room temperature (or the temperature of the solution system when washing the material) by calculation or measurement; then measure the mass of the residual lithium source in the material to be washed Fraction x; finally, dissolve y g lithium source in V mL pure water to prepare washing solution. The physical parameters in this method satisfy y=0.01Vs-mx, wherein m is the total mass of the material to be washed, and both x and y are in LiOH equivalent.
[0031] Take 20g of the material to be washed and add it to 50mL of the above washing liqu...
Embodiment 2
[0033] With Ni 0.8 co 0.15 Al 0.05 (OH) 2 As the precursor, with LiOH·H 2 O is the lithium source (slightly excessive), and the high-nickel cathode material LiNi is prepared by a high-temperature solid-phase method. 0.8 co 0.15 Al 0.05 o 2 , to be washed. Configure LiOH washing solution: obtain the solubility s (g / 100g water) of LiOH in pure water at the current room temperature (or the temperature of the solution system when washing the material) by calculation or measurement; then measure the mass of the residual lithium source in the material to be washed Fraction x; then prepare saturated LiOH solution a mL; finally add pure water b mL to the above solution to obtain a washing solution. The physical parameters described in the method satisfy mx=0.01sb, and x is in LiOH equivalent.
[0034] Take 20g of the material to be washed and add it to 50mL of washing liquid and stir it magnetically for 5min, then separate the solid and liquid, wash and dry to obtain the wash...
Embodiment 3
[0036] With Ni 0.8 co 0.15 Al 0.05 (OH) 2 As the precursor, with LiOH·H 2 O is the lithium source (slightly excessive), and the high-nickel cathode material LiNi is prepared by a high-temperature solid-phase method. 0.8 co 0.15 Al 0.05 o 2 , to detect the amount of residual lithium in the material to be washed, to be washed. Configure LiOH washing solution: obtain the solubility s of LiOH in pure water at the current room temperature by calculation or measurement 1 (g / 100g water); then measure and calculate the mass fraction x of the residual lithium source in the material to be washed; then prepare a saturated LiOH solution a mL; finally raise the temperature of the saturated LiOH solution to increase its solubility to s 2 , to obtain the wash solution. The physical quantity described in this method satisfies mx+0.01s 1 a=0.01s 2 a, x is in LiOH equivalent.
[0037] Take 20g of the material to be washed and add it to the above washing solution, stir it magneticall...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com