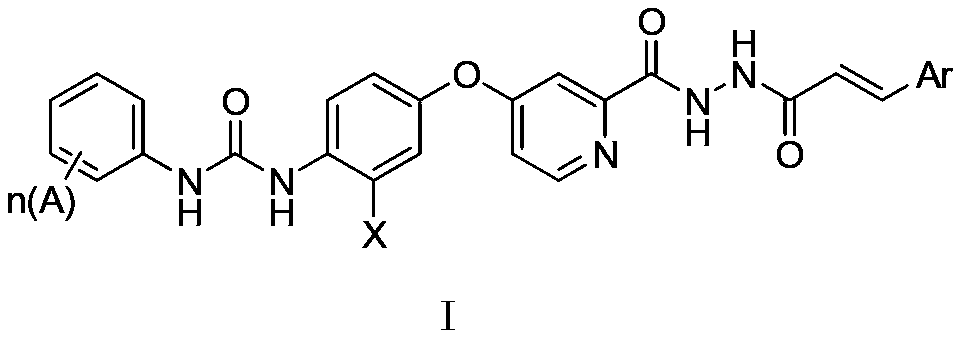
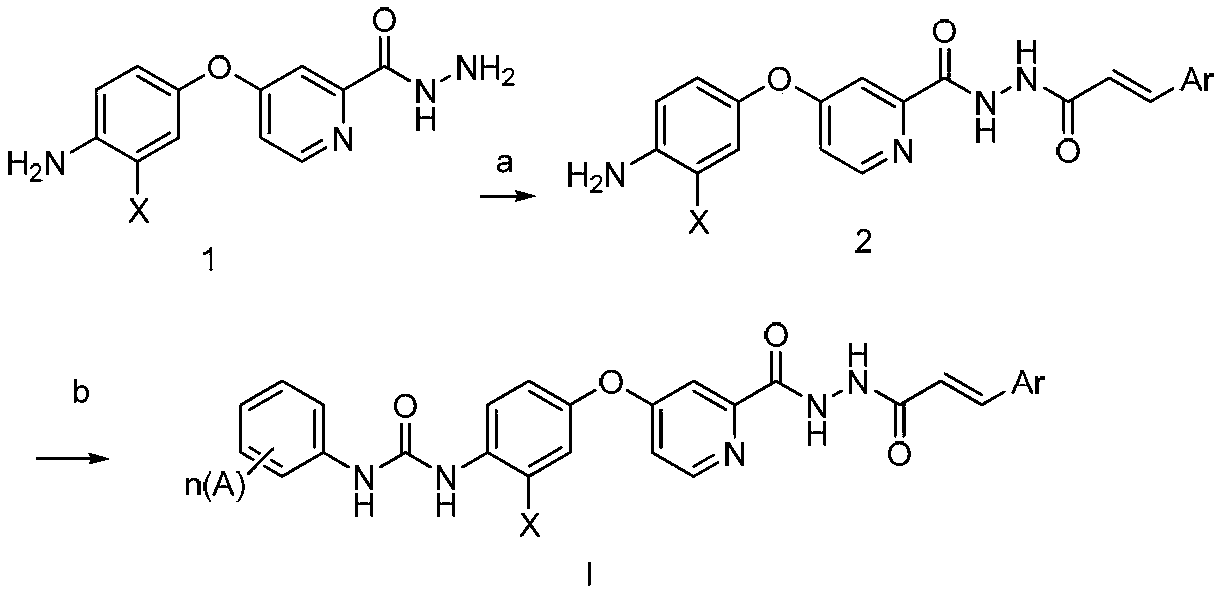
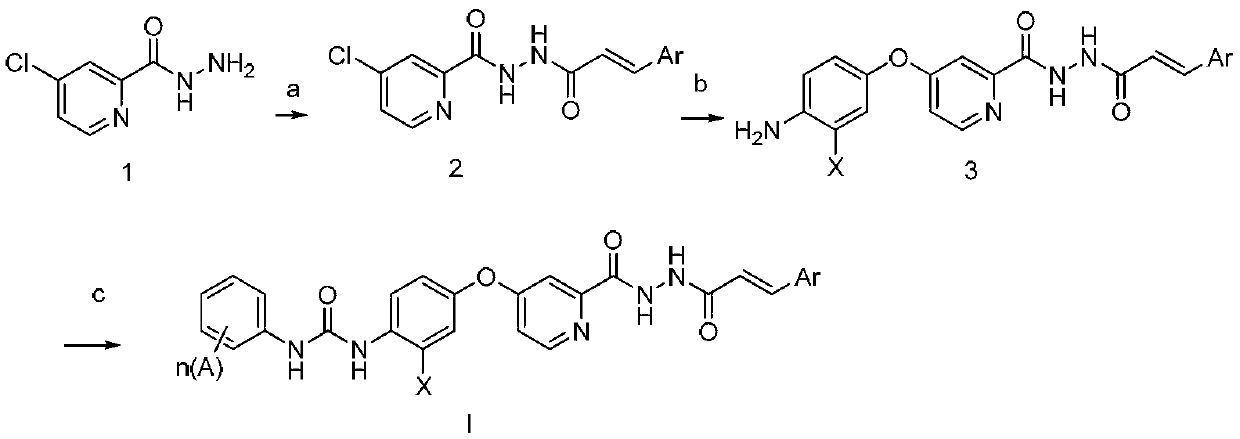
N'-Aroyl o-pyridine hydrazide derivatives and its preparation method, pharmaceutical composition and application
A kind of technology of pyridine hydrazide and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1.1-(4-(2-(2-(3-p-chlorophenylacryloyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoromethyl Phenyl)urea
[0074]
[0075] Synthesis of N'-(3-p-chlorophenylacryloyl)-4-(p-aminophenoxy)pyridine-2-hydrazide
[0076] Dissolve 105mg (0.43mmol) of compound 4-(p-aminophenoxy)pyridine-2-hydrazide in 7ml THF, add 0.09mL (0.64mmol) TEA, dropwise add 2ml dissolved in 0.52mmol 3-p-chlorophenylacryloyl chloride After refluxing for 4 hours, a white solid was formed, the reaction was stopped, filtered, washed with a small amount of tetrahydrofuran, washed with water, and dried to obtain N'-(3-p-chlorophenylacryloyl)-4-(p-aminophenoxy Base) pyridine-2-hydrazide, white solid 60mg. 1 H NMR (400MHz, DMSO-d 6 ):10.59(br,1H,-CONH 2 -),10.48(s,1H,-CONH-),8.52(d,1H,Ar-H),7.56(m,3H,Ar-H),7.49(m,4H,Ar-H),6.88(d ,2H,Ar-H),6.73(d,1H,-CH=CH-Ar),6.65(d,1H,Ar-H),5.19(s,2H,Ar-NH 2 ).MS(FAB):(M + +1=409).
[0077] 1-(4-(2-(2-(3-p-chlorophenylacryloyl)hydrazinecarb...
Embodiment 2
[0082] Example 2.1-(4-(2-(2-(3-trifluoromethylphenylacryloyl)hydrazine carbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-tri Fluoromethylphenyl) urea
[0083]
[0084] Using 3-m-trifluoromethylphenylacrylic acid instead of 3-p-chlorophenylacrylic acid, the procedure of Example 1 was followed to obtain 175 mg of the target compound as a white solid. 1 H NMR (400MHz, DMSO-d 6 ):10.65(m,2H,-CONH-),9.39(s,1H,-CONH-),9.16(s,1H,-CONH-),8.57(d,1H,Ar-H),8.03(m, 3H,Ar-H),7.61(m,7H,Ar-H,=CH-Ph),7.40(s,1H,Ar-H),7.20(m,3H,Ar-H),6.86(d,1H ,-COCH=).MS(FAB)(M + +1=664)
Embodiment 3
[0085] Example 3.1-(4-(2-(2-(3-o-p-dichlorophenylacryloyl)hydrazinecarbonyl)pyridine-4-oxyl)phenyl)-3-(4-chloro-3-trifluoro Methylphenyl) urea
[0086]
[0087] Using 3-o-p-dichlorophenylacrylic acid instead of 3-p-chlorophenylacrylic acid, and referring to the operation of Example 1, 135 mg of the target compound was obtained as a white solid. 1 H NMR (400MHz, DMSO-d 6 ):10.64(br,2H,-CONH-),9.46(s,1H,-CONH-),9.23(s,1H,-CONH-),8.56(d,1H,Ar-H),8.12(s, 1H,Ar-H),7.87(m,1H,Ar-H),7.72(m,3H,Ar-H,=CHPh),7.40(m,3H,Ar-H),7.40(m,3H,Ar -H),6.78(d,1H,-COCH=);MS(FAB)(M + +1=664)
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com