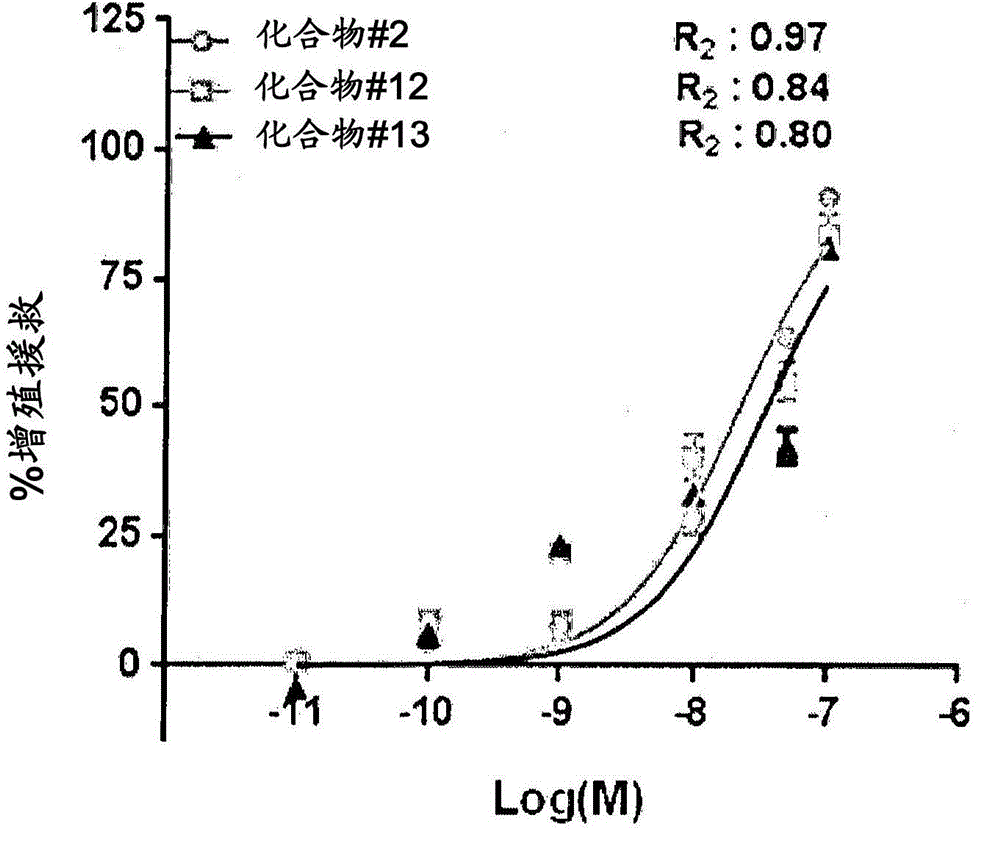
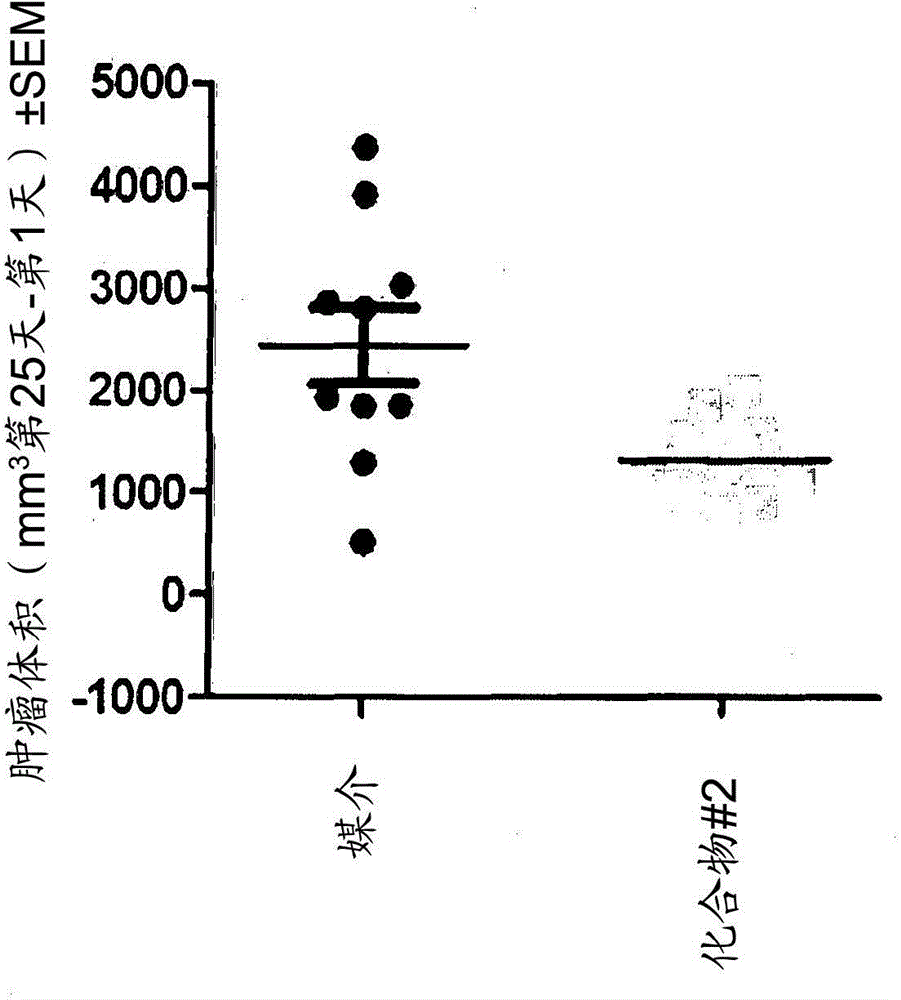
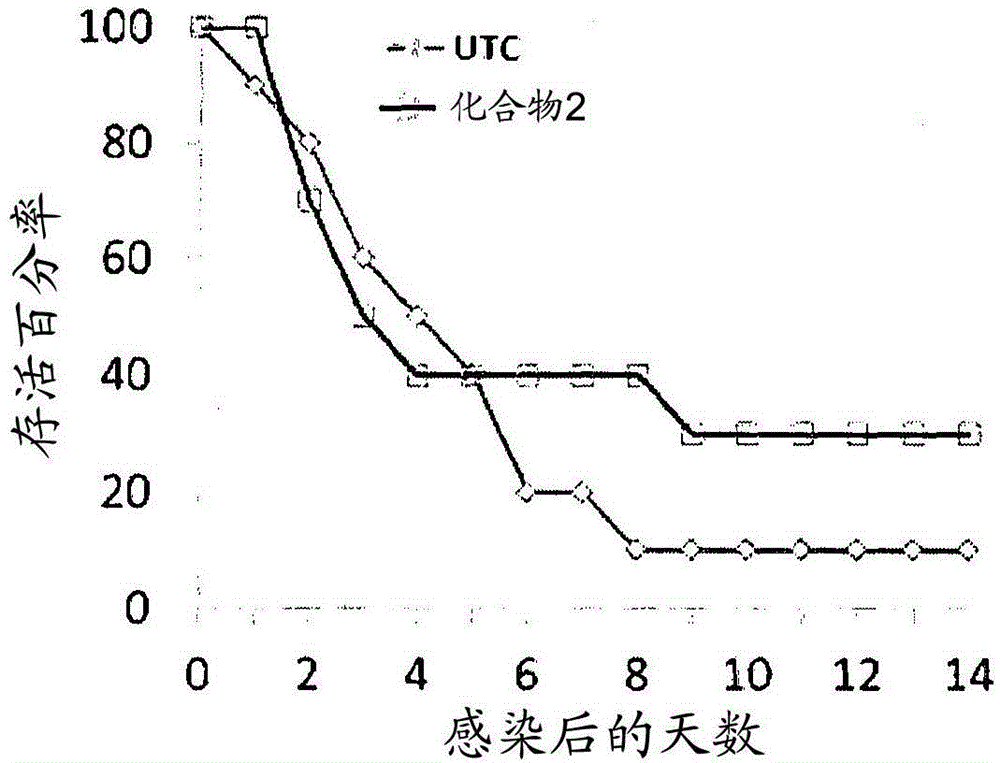
Peptidomimetic compounds as immunomodulators
A compound, selected technology, applied in the fields of peptides, organic chemistry, drug combination, etc., can solve the problem of peptidomimetic compounds without PD-1 immunomodulator
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0195] Example 1: Synthesis of Compound 1
[0196]
[0197] step 1:
[0198]
[0199] Diazomethane (generated from 8g N-nitroso-N-methylurea in 50% aqueous KOH) in ether was added to compound 1a (6g, 23.0mmol) at 0°C under a nitrogen atmosphere. MeOH (60 mL) solution, and the reaction was stirred at room temperature for 30 min. The reaction was monitored by TLC. After the reaction was completed, the reaction mixture was concentrated under reduced pressure to produce 5.9 g of pure compound 1b (yield: 93%).
[0200] LCMS: 220.1[(M-O t Bu)+H]) + , 298.2(M+Na) + .
[0201] Step 2:
[0202]
[0203] 99% hydrazine hydrate (1 mL) was added to a solution of intermediate 1b (1.0 g, 3.6 mmol) in methanol (10 mL), and stirred at room temperature for 12 h. The completion of the reaction was confirmed by TLC analysis. The reaction mixture was evaporated under reduced pressure to produce 0.75 g of pure compound 1c (yield: 75.0%).
[0204] LCMS: 276.3 (M+H) + .
[0205] Step 3:
[0206]
[0207] The...
Embodiment 2
[0215] Example 2: Synthesis of Compound 2
[0216]
[0217] Method A:
[0218] Step 1a:
[0219]
[0220] The bonding of urea was performed at room temperature using the coupling of compound 1f (5 g, 7.9 mmol) and compound 2f (3.36 g, 9.5 mmol) in THF (25 mL). The coupling was initiated by adding TEA (1.6 g, 15.8 mmol) in THF (25 mL), and the resulting mixture was stirred at room temperature. After the completion of 20h, THF was evaporated from the reaction mass and partitioned between water and ethyl acetate. The organic layer was washed with water, brine, and passed through Na 2 SO 4 It was dried and evaporated under reduced pressure to produce compound 2a, and the residue was additionally washed with ether / hexane (7:3) to produce 3.0 g of compound 2a. LCMS: 923.8 (M+H) + .
[0221] Step 2a:
[0222]
[0223] Under an inert atmosphere, to a solution of intermediate 2a (0.5 g) in methanol (10.0 mL) was added 10% Pd-C (0.1 g), and the mixture was heated at room temperature in H 2 St...
Embodiment 3
[0252] Example 3: Synthesis of Compound 3
[0253]
[0254] To intermediate 2j (0.92g, 1.0mmol) in CH 2 Cl 2 The solution in (5mL) was added with trifluoroacetic acid (5mL) and a catalytic amount of triisopropylsilane, and stirred at room temperature for 3h to remove acid-sensitive protecting groups. The resulting solution was concentrated in vacuo to produce 0.3 g of compound 3 as a crude solid. LCMS: 469.1 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com