Pharmaceutical composition for treating chloasma and preparation method thereof
A composition and technology of chloasma, applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of unsatisfactory treatment effect, toxic and side effects, etc., and achieve the reduction of lipid peroxidation and the curative effect Definitely, the effect of reducing the production of melanin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
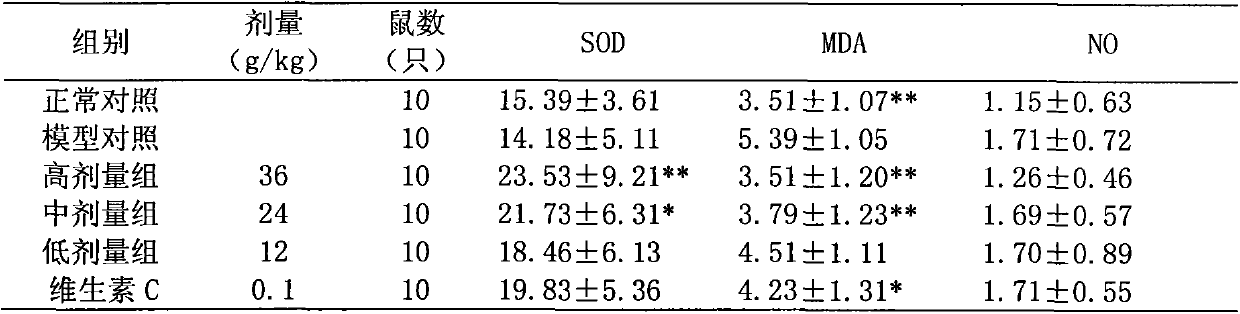
[0063] The raw materials were weighed according to the following proportions: 50 parts of Ligustrum lucidum, 50 parts of Eclipta chinensis, 80 parts of Salvia miltiorrhiza, 70 parts of angelica, 15 parts of cinnamon, 15 parts of rose, and 5 parts of grape seed extract.
[0064] Preparation method: according to the above-mentioned raw materials and weight ratio of the traditional Chinese medicine composition, the medicinal materials are respectively taken, firstly add Ligustrum lucidum, eclipta, salvia miltiorrhiza, angelica, cinnamon, and roses into the extraction tank, soak for 30 minutes for the first time, add Decoct 10 times the amount of water twice, each time for 1.5h, filter, and combine the filtrates; add 70% ethanol, precipitate, and obtain a supernatant, which is concentrated under reduced pressure (70°C, 0.08Mpa) to a relative density of 1.08 ~1.10 (60°C), recover ethanol to obtain extract, then vacuum dry (70°C, 0.08Mpa), pulverize, and pass through a 60-mesh sieve ...
Embodiment 2
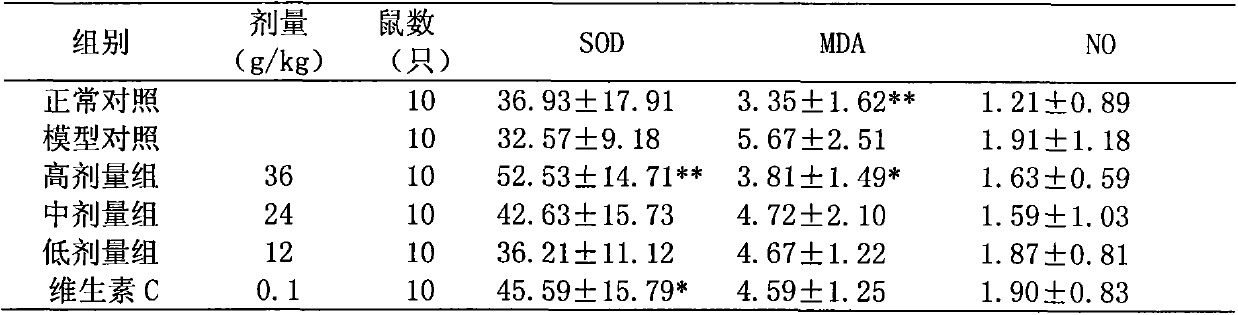
[0066] The raw materials were weighed according to the following proportions: 10 parts of Ligustrum lucidum, 10 parts of Eclipta chinensis, 30 parts of Salvia miltiorrhiza, 20 parts of angelica, 5 parts of cinnamon, 5 parts of rose, and 0.1 part of grape seed extract.
[0067] Preparation method: according to the above-mentioned raw materials and weight ratio of the traditional Chinese medicine composition, the medicinal materials are respectively taken, firstly add Ligustrum lucidum, eclipta, salvia miltiorrhiza, angelica, cinnamon, and roses into the extraction tank, soak for 30 minutes for the first time, add Decoct 10 times the amount of water twice, each time for 1.5h, filter, and combine the filtrates; add 70% ethanol, precipitate, and obtain a supernatant, which is concentrated under reduced pressure (70°C, 0.08Mpa) to a relative density of 1.08 ~1.10 (60°C), recover ethanol to obtain extract, then vacuum dry (70°C, 0.08Mpa), pulverize, and pass through a 60-mesh sieve t...
Embodiment 3
[0069] The raw materials were weighed according to the following proportions: 30 parts of Ligustrum lucidum, 30 parts of Eclipta chinensis, 60 parts of Salvia miltiorrhiza, 40 parts of angelica, 10 parts of cinnamon, 10 parts of rose, and 2 parts of grape seed extract.
[0070]Preparation method: according to the above-mentioned raw materials and weight ratio of the traditional Chinese medicine composition, the medicinal materials are respectively taken, firstly add Ligustrum lucidum, eclipta, salvia miltiorrhiza, angelica, cinnamon, and roses into the extraction tank, soak for 30 minutes for the first time, add Decoct 10 times the amount of water twice, each time for 1.5h, filter, and combine the filtrates; add 70% ethanol, precipitate, and obtain a supernatant, which is concentrated under reduced pressure (70°C, 0.08Mpa) to a relative density of 1.08 ~1.10 (60°C), recover ethanol to obtain extract, then vacuum dry (70°C, 0.08Mpa), pulverize, and pass through a 60-mesh sieve t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com