Berberine derivative and use thereof
A technology for berberine and its use, which is applied in the field of berberine derivatives and their preparation, and can solve the problems of unseen effects on glioma migration and invasion, and unsatisfactory drug efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
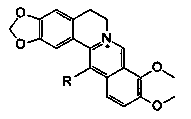
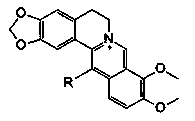
[0024] 1. Synthesis and identification of 13-substituted berberine derivatives
[0025]
[0026] 1.1 Synthesis and identification of intermediate dihydroberberine (Dihydroberberine, 1b)
[0027] Intermediate 1b was prepared by reduction method.
[0028] Dry 16g (0.0162mol) was suspended in 50mL pyridine and stirred at room temperature. Add NaBH slowly 4 700 mg, when it turns into a reddish-brown clear solution, immediately add 200 mL of ice water to the reaction solution, filter to obtain intermediate 1b, which appears as a light yellow powder, and vacuum-dry at 30°C for use. ESI-MS m / z :338[M+H] + .
[0029] 1. Synthesis and identification of 1.213-decyl berberine (B7)
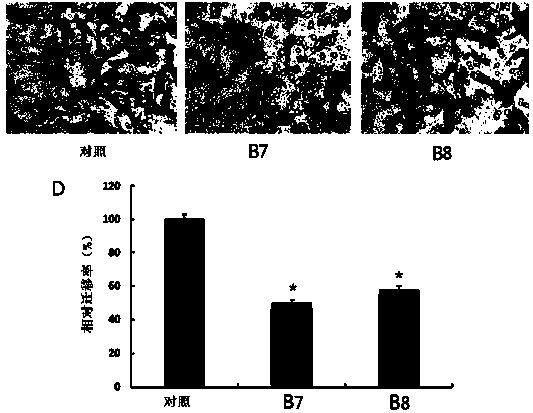
[0030] Bromidedecane 0.89mL (4e.q. is 4 times the molar amount of 1b), sodium iodide (NaI) 0.60g (4e.q.), anhydrous acetonitrile 20mL, placed in a sealed high-pressure reaction bottle, Reaction at 80°C for 24h. Add 1b0.34g (1mmol, 1e.q.), fill with N 2 15min, reaction 12h. Afterwards, heat and r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com